Key Results
Key Result 1
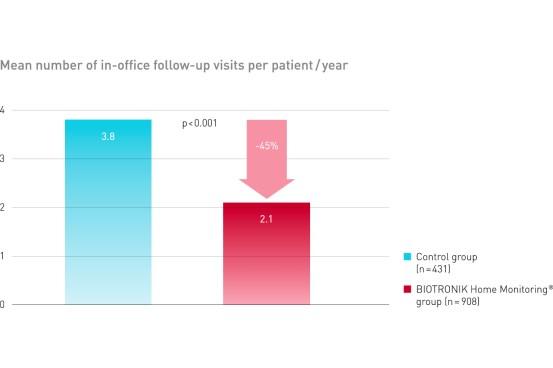
TRUST demonstrated equal safety event rate in both groups. BIOTRONIK Home Monitoring delivered a reduction of 45% of in-office follow-up visits.
Efficacy and Safety of Automatic Remote Monitoring for Implantable Cardioverter-Defibrillator Follow-Up
Varma, Circulation 2012
TRUST demonstrated equal safety event rate in both groups. BIOTRONIK Home Monitoring delivered a reduction of 45% of in-office follow-up visits.

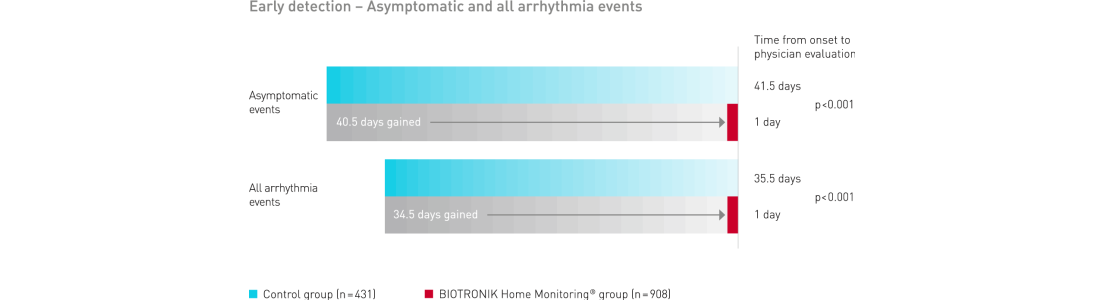
BIOTRONIK Home Monitoring delivered a significant gain in early detection of clinically relevant symptomatic and asymptomatic events
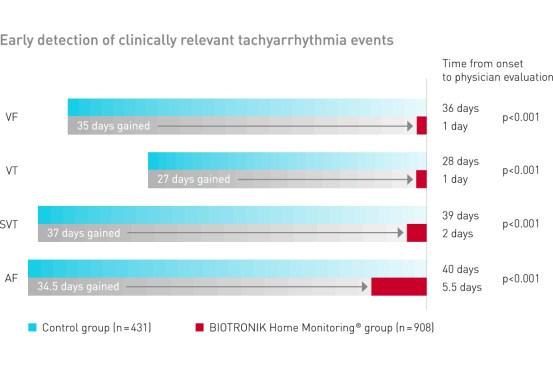
BIOTRONIK Home Monitoring significantly reduced the time to evaluation of clinically relevant tachyarrhythmia events
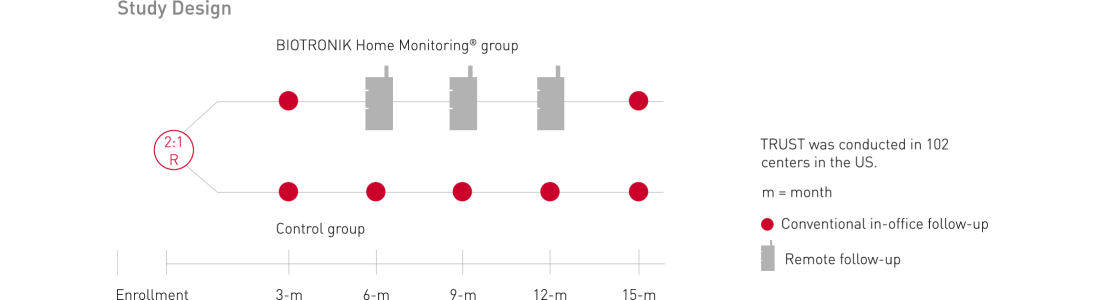
Patient participation and follow up period: up to 15 months
NCT00336284