Key Results
Key Result 1
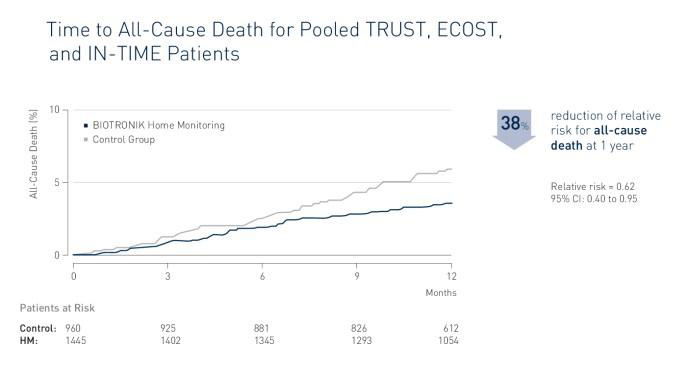
BIOTRONIK Home Monitoring is associated with a significant reduction of all-cause mortality. The study TRUECOIN presents a relative risk reduction of 38%. The absolute reduction of all-cause mortality is 1.9%.
Daily Remote Monitoring of Implantable Cardioverter-Defibrillators – Pooled Individual Patient Data from IN-TIME, ECOST, and TRUST Trials Suggest a Mechanism of Clinical Benefit
Hindricks et al., European Heart Journal 2017
BIOTRONIK Home Monitoring is associated with a significant reduction of all-cause mortality. The study TRUECOIN presents a relative risk reduction of 38%. The absolute reduction of all-cause mortality is 1.9%.

BIOTRONIK Home Monitoring is associated with a significant reduction of all-cause mortality or worsening of heart failure (WHF) hospitalization. TRUECOIN presents a relative risk reduction of 36% of all-cause death or WHF hospitalization. The absolute reduction of all-cause death or WHF hospitalization is 5.6%.
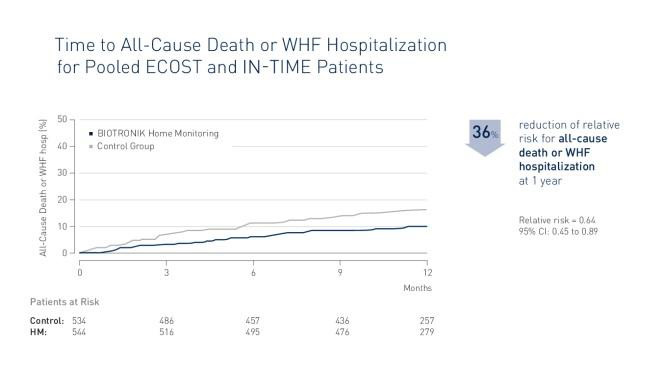
The similar magnitude of absolute risk reduction for WHF and cardiovascular endpoints suggests that the benefit of Home Monitoring is driven by the prevention of heart failure exacerbation.
1 Cowie MR; Remote management of heart failure using implanted devices and formalized follow-up procedures (REM-HF); ESC 2016.; 2 Parthiban N, Esterman A, Mahajan R, Twomey DJ, Pathak RK, Lau DH, Roberts-Thomson KC, Young GD, Sanders P, Ganesan AN; Remote Monitoring of Implantable Cardioverter-Defibrillators: A Systematic Review and Meta-Analysis of Clinical Outcomes; J Am Coll Cardiol. 2015 Jun 23; 65(24):2591-600.