BIOMAG-I 12-Month Study Data Highlights Continued Excellent Patient Outcomes With New DREAMS 3G Scaffold Safety and Performance of BIOTRONIK’s New Generation Resorbable Magnesium Scaffold at 12 Months Highlighted at EuroPCR
In the first-in-human study BIOMAG-I, BIOTRONIK’s new-generation DREAMS 3G resorbable magnesium scaffold (RMS) showed significantly lower in-scaffold late lumen loss (LLL) than its predecessor at 12 months as well as excellent safety and efficacy. Prof. Michael Haude, BIOMAG-I Coordinating Clinical Investigator, presented the latest results in the late breaking trial session at the EuroPCR course.1
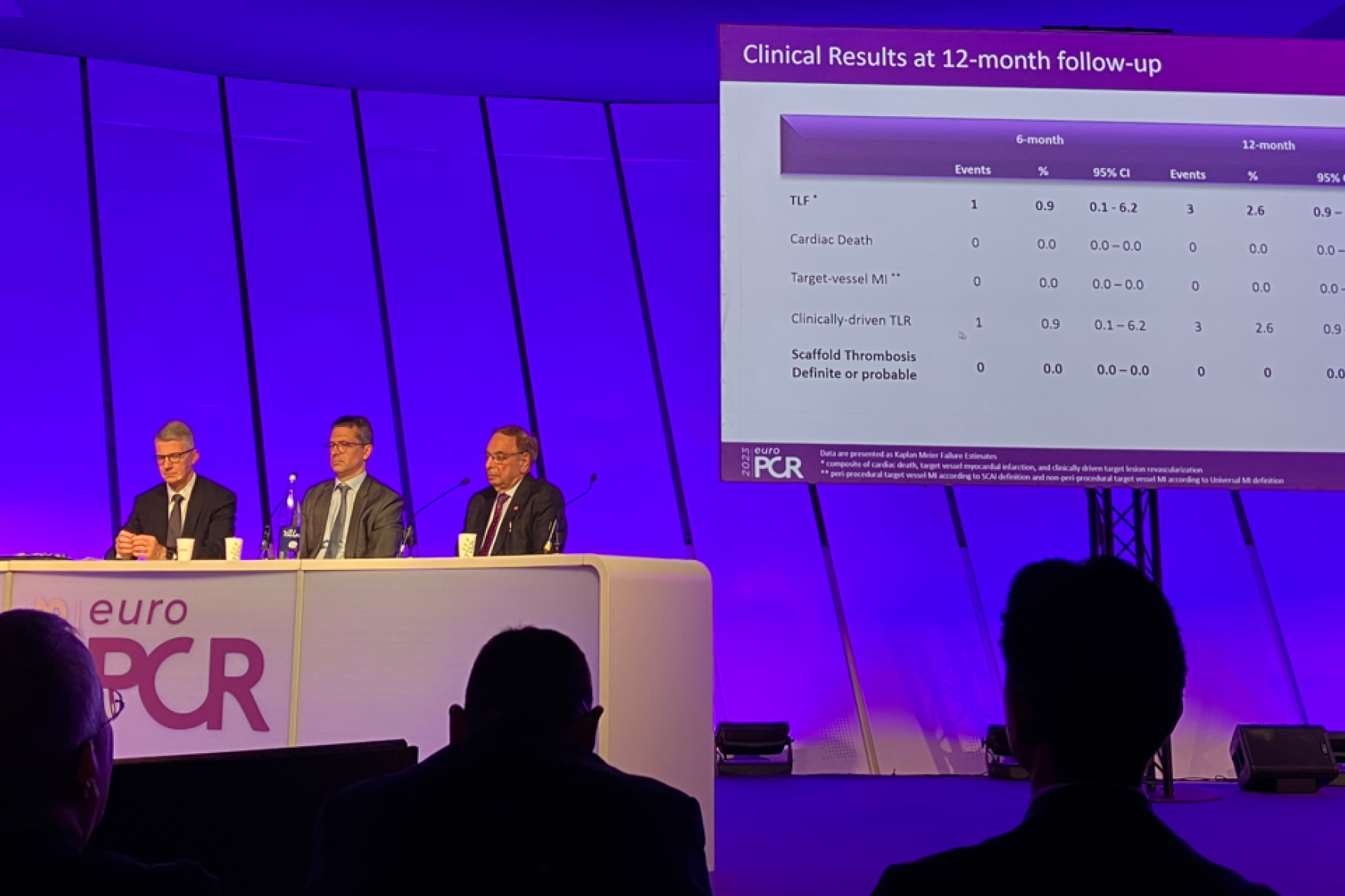
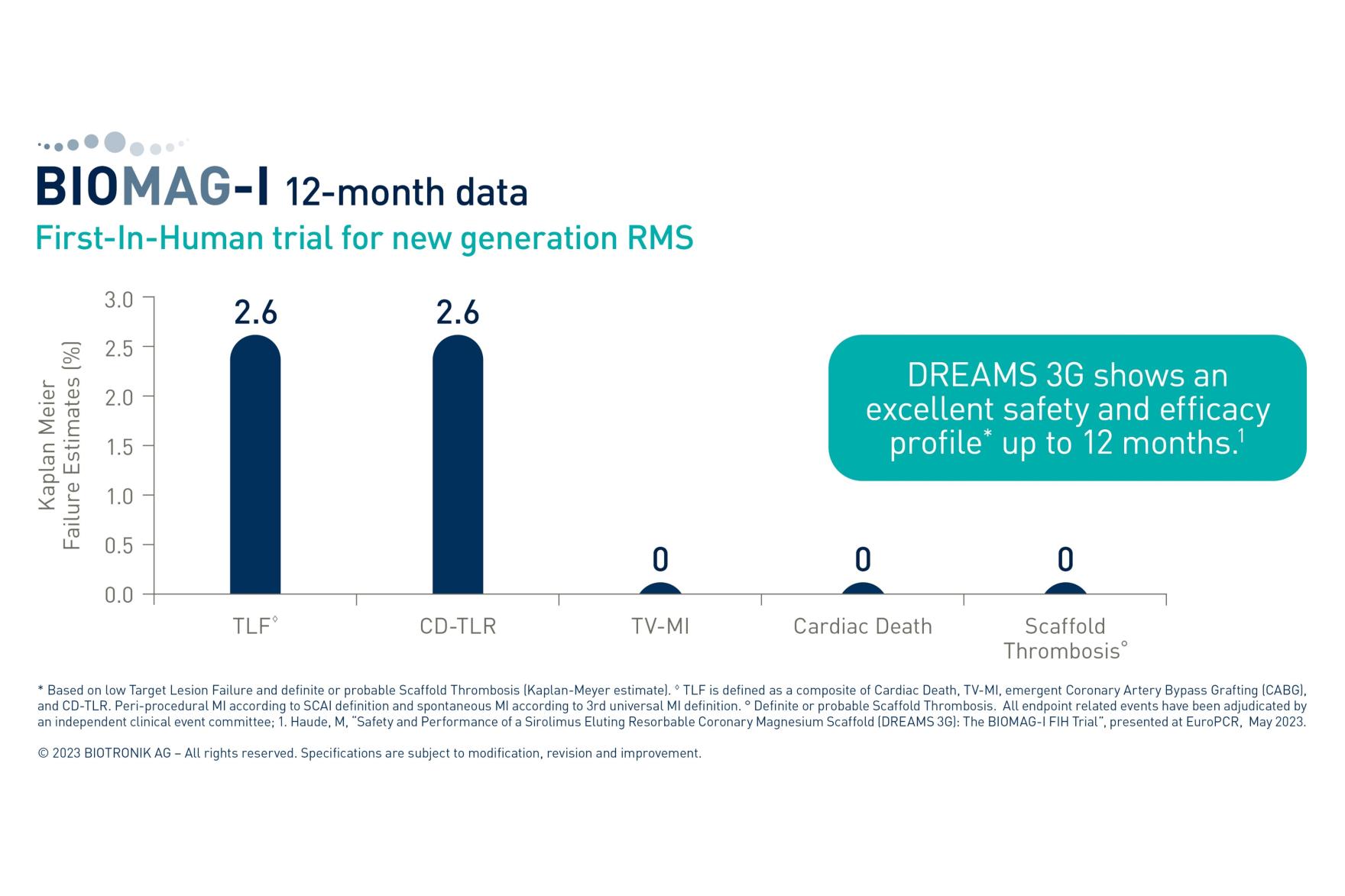
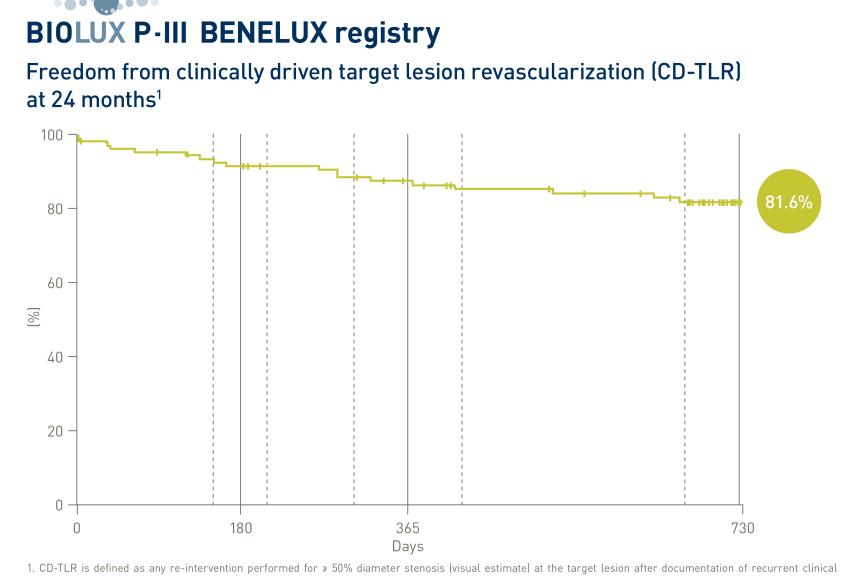
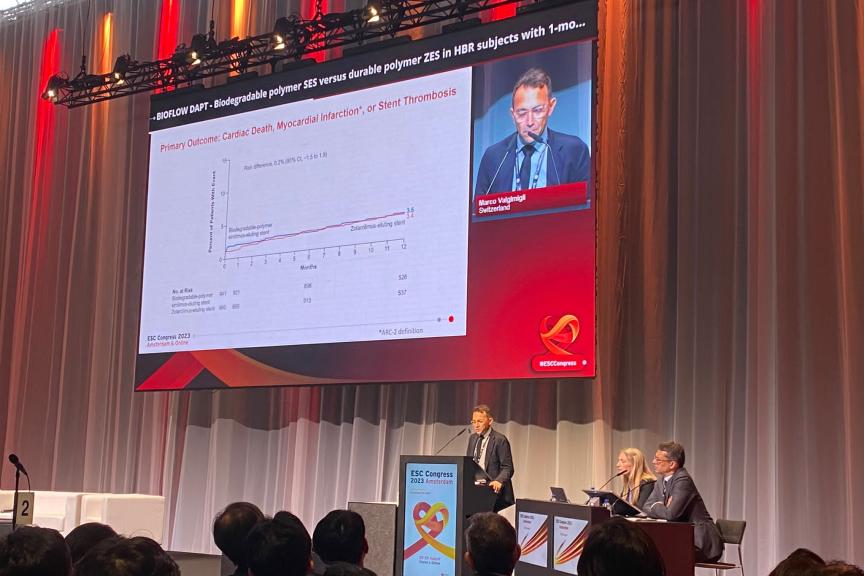
At one-year follow-up, BIOMAG-I data confirmed the excellent safety profile of DREAMS 3G RMS with a low target lesion failure rate of 2.6%. Neither cardiac death and myocardial infarction occurred, nor scaffold thrombosis was reported. After completion of the resorption, the in-scaffold LLL was 0.24±0.36 mm, comparable to contemporary drug-eluting stents (DES).2
“At the end of the scaffold resorption, at 12 months the data showed, that the DREAMS 3G scaffold has an excellent safety and efficacy profile”, said Prof Michael Haude, Rheinland Klinikum, Germany. “The late lumen loss is not just 38% better than the predecessor3 but also on a level of a contemporary DES making it a valuable alternative to DES.”2
The prospective BIOMAG-I clinical trial assesses the angiographic, clinical and safety performance of DREAMS 3G RMS of 116 patients with single de novo lesions in up to two coronary arteries. A total of 14 clinics in eight European countries are taking part. 20% of the patients presented with NSTEMI and more than 75% with B2/C lesions. The ‘4P’-protocol for RMS implantation – patient selection, adequate pre-dilatation, proper sizing and adequate post-dilatation – was adhered to.
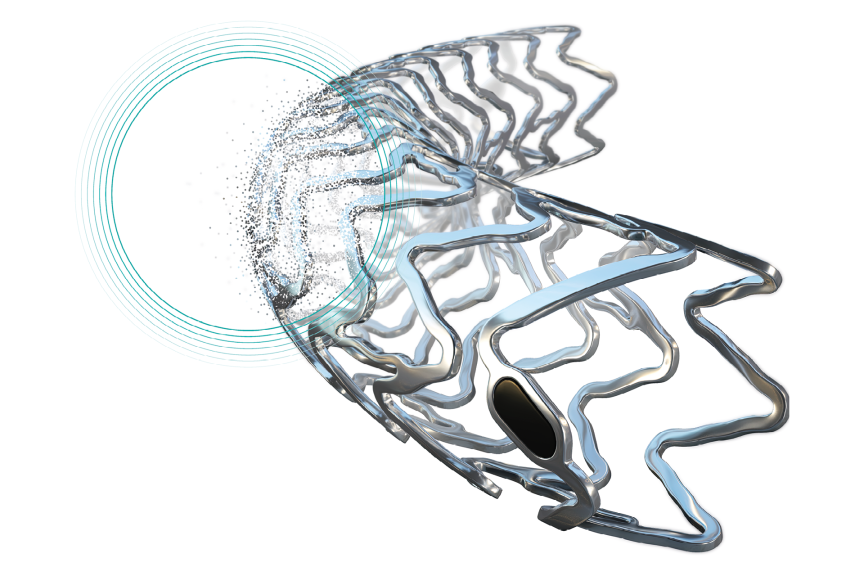
Bioresorbable scaffolds have been developed to provide initial ‘stent-like’ mechanical support to the vessel but thereafter disappear and thus prevent long-term stent-related adverse events.4 DREAMS 3G RMS is made of BIOTRONIK’s proprietary BIOmag® magnesium alloy and maintains a resorption time of 12 months. Compared to the DREAMS 2G predecessor device, it offers unique benefits such as reduced strut thickness and longer vessel support.5,6
“The late lumen loss suggests that DREAMS 3G RMS provides a good vessel support over time”, said Prof. Dr. Georg Nollert, Vice President Medical Affairs, Vascular Intervention at BIOTRONIK. “Having a resorbable scaffold that is as effective as contemporary drug-eluting stents but resorbs over time means that we can provide physicians and patients with a true alternative. This treatment option offers an ‘implant-free future’ from which especially younger patients can benefit.”
The BIOMAG-I 12-month-results were simultaneously published as a fast-track publication in the EuroIntervention Journal.
-END-
References:
1. Haude M., BIOMAG-I: one-year clinical outcome of the resorbable magnesium scaffold-DREAMS 3G, presented at EuroPCR 2023.
2. Byrne RA, Serruys PW, Baumbach A, Escaned J, Fajadet J, James S, Joner M, Oktay S, Jüni P, Kastrati A, Sianos G, Stefanini GG, Wijns W, Windecker S- Report of a European Society of Cardiology-European Association of Percutaneous Cardiovascular Interventions task force on the evaluation of coronary stents in Europe: executive summary. Eur Heart J 2015;36:2608-2620.
3. Haude M, et al, Sustained safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de novo coronary lesions: 12-month clinical results and angiographic findings of the BIOSOLVE-II first-in-man trial. Eur Heart J 2016;37:2701-2709.
4. Haude M. et al, Safety and performance of the third-generation drug-eluting resorbable coronary magnesium scaffold system in the treatment of subjects with de novo coronary artery lesions: 6-month results of the prospective, multicenter BIOMAG-I first-in-human study, published in The Lancet (Vol 59, May 2023). DOI: https://doi.org/10.1016/j.eclinm.2023.101940.
5. Seguchi et al. Preclinical evaluation of the degradation kinetics of third generation resorbable magnesium scaffolds. EuroIntervention, published online 2023. DOI: 10.4244/EIJ-D-22-00718
6. BIOTRONIK data on file.
Disclaimer:
DREAMS 3G is commercially not available.
BIOmag and DREAMS are trademarks or registered trademarks for the BIOTRONIK Group of Companies. All other trademarks are the property of their respective owners.
About BIOTRONIK
At BIOTRONIK, patient well-being is our top priority and has been for 60 years. BIOTRONIK is a leading global medical technology company with products and services that save and improve the lives of millions suffering from heart and blood vessel diseases as well as chronic pain. Driven by a purpose to perfectly match technology with the human body, we are dedicated innovators who develop trusted cardiovascular, endovascular and neuromodulation solutions. BIOTRONIK is headquartered in Berlin, Germany, and is represented in over 100 countries.