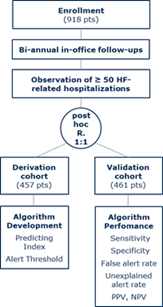
Key Result
The algorithm predicted heart failure hospitalizations early, with high sensitivity and with a low false alert rate
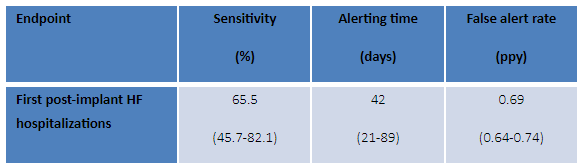
Table 1: Main performance parameters of the predicting algorithm
(Numbers in brackets: confidence intervals, except for alerting time (IQR).