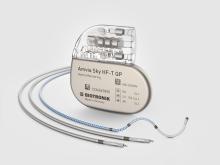
Rivacor 5 HF-T QP/HF-T
Melhorando o tratamento e reduzindo riscos por até 15 anos 9
Para pacientes com arritmias, a terapia com o desfibrilador cardioversor implantável pode ser um salva-vidas, minimizando riscos e aumentando a qualidade de vida a longo prazo. Isso é o que os menores, mais novos e mais simples sistemas Rivacor 5 ICD foram desenvolvidos para proporcionar, por até 15 anos9 — otimizando o tratamento onde e quando necessário. Quando associado à tecnologia Home Monitoring®, a fibrilação atrial pode ser detectada mais cedo14 e as taxas de choques inapropriados e hospitalizações podem ser reduzidas19. E isso foi clinicamente provado.
Destaques do produto
BIOshape. Desfibrilador cardioversor implantável ultrafino de 10 mm
Os desfibriladores cardioversores implantáveis Rivacor são pequenos e finos – apenas 10 mm de uma ponta à outra – e dispõem de um formato (BIOshape1) liso, elíptico e anatômico.
15 anos de longevidade. 10 anos de garantia
A bateria dos desfibriladores cardioversores implantáveis Rivacor têm uma vida útil estendida de até 15 anos9 e uma garantia total de 10 anos.
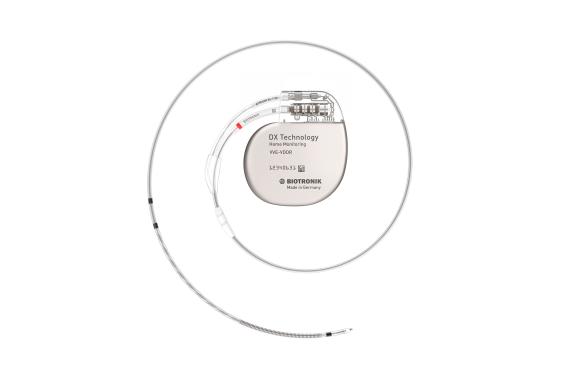
Tecnologia DX
Todos esses benefícios também estão disponíveis na exclusiva tecnologia DX, que proporciona diagnósticos atriais completos com um único eletrodo.
Downloads e links relacionados
Contato
Referências
1 Contoured housing; Acticor/Rivacor VR: 60 x 61.5 x 10 mm; 30 ccm
2 as part of an MR Conditional system
3 Post-Market observation; interim analysis, December 21, 2018. Data on file
4 Device shape analysis, February 2019. Data on file.
5 Post-market observation; interim analysis, December 21, 2018. Data on file.
6 Post-market observation; interim analysis, December 21, 2018. Data on file.
7 Device shape analysis, February 2019. Data on file.
8 Post-market observation; interim analysis, December 21, 2018. Data on file.
9 Single-Chamber ICD standard conditions. Data on file (service time calculation)
10 Acticor/Rivacor Single-Chamber ICD @ 60 ppm, 15% pacing, 2.5V, 500 Ohms: Medtronic 3T FBS; VISIA AF (EVERA; MIRRO) MRI S VR SureScan:
10.7 years (MDT IFU) vs Acticor 7 VR-T ProMRI: 14.9 years. Competitor device manuals as of Nov. 2018
11 Polyzos KA, Konstantelias AA, Falagas ME, Risk factors for cardiac implantable electronic device infection: A systematic review and meta-analysis,
Europace (2015) 17, 767-777.
12 Fact file: Cardiac Imaging with MRI, CT and Nuclear Techniques British Heart Foundation. January 2010.
13 When patients are monitored by BIOTRONIK Home Monitoring. See ProMRI(R) manual for all details.
14 Varma N et al. Efficacy and Safety of Automatic Remote Monitoring for Implantable Cardioverter-Defibrillator Follow-Up:
The Lumos-T Safely Reduces Routine Office Device Follow-Up (TRUST) Trial. Circulation, 2010; 122: 325–332.
15 Guedon-Moreau L et al. Decreased delivery of inappropriate shocks achieved by remote monitoring of ICD: a substudy of the ECOST trial.
J Cardiovasc Electrophysiol, 25 (2014), 763-770.
16 Guedon-Moreau L et al. Decreased delivery of inappropriate shocks achieved by remote monitoring of ICD: a substudy of the ECOST trial.
J Cardiovasc Electrophysiol, 25 (2014), 763-770.
17 Varma N et al. Efficacy and Safety of Automatic Remote Monitoring for Implantable Cardioverter-Defibrillator Follow-Up:
The Lumos-T Safely Reduces Routine Office Device Follow-Up (TRUST) Trial. Circulation, 2010; 122: 325–332.
18 Schwab JO et al. Clinical Course of Dual-Chamber Implantable Cardioverter-Defibrillator Recipients followed by Cardiac Remote Monitoring:
Insights from the LION Registry. BioMed Research International, 2018, https://doi.org/10.1155/2018/3120480.
19 Guedon-Moreau L et al. Decreased delivery of inappropriate shocks achieved by remote monitoring of ICD: a substudy of the ECOST trial.
J Cardiovasc Electrophysiol, 25 (2014), 763-770.
20 Guedon-Moreau L et al. Decreased delivery of inappropriate shocks achieved by remote monitoring of ICD: a substudy of the ECOST trial.
J Cardiovasc Electrophysiol, 25 (2014), 763-770.
21 Ricci R P et al. Long-term patient acceptance of and satisfaction with implanted device remote monitoring. Europace (2010) 12, 674-679.
22 Ricci R P et al. Long-term patient acceptance of and satisfaction with implanted device remote monitoring. Europace (2010) 12, 674-679.
23 Hindricks G et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomized controlled trial.
The Lancet. 2014; 384 (9943): 583-590
24 vs. 55% - Crossley G H et al. The CONNEXT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) Trial.
JACC. 2011; 57(10):1181-1189 [for bar chart comparison only]
25 Ricci R P et al. Long-term patient acceptance of and satisfaction with implanted device remote monitoring. Europace (2010) 12, 674-679.
26 Performance analysis. Data on file, 2018
27 Performance analysis. Data on file, 2018
28 Kurt M et al. Avoiding inappropriate therapy of single-lead implantable cardioverter defibrillator by atrial-sensing electrodes. Journal of Cardiovasc.
Electrophysiol. 2018; 29(12): 1682-1689