Key Results
Key Result 1
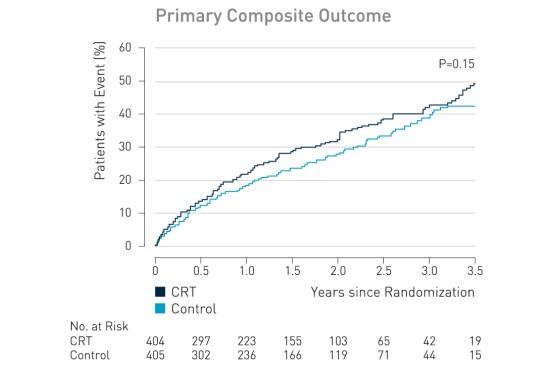
The primary outcome, death from any cause or hospitalization for worsening heart failure, occurred in 116 of 404 patients (28.7%) in the CRT group, as compared with 102 of 405 (25.2%) in the control group (hazard ratio with CRT, 1.20; 95% confidence interval [CI], 0.92 to 1.57; P = 0.15).