Freesolveᵀᴹ
Indicated for de novo coronary artery lesionsa
Support
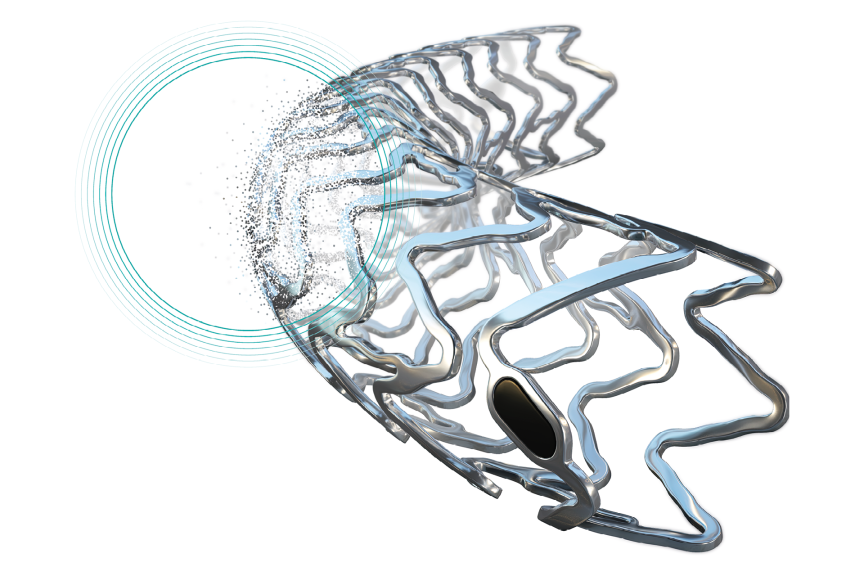
Resorbable coronary scaffolds widen coronary artery stenoses and provide temporary vessel support. Thereby, scaffolds enable unobstructed blood flow in the coronary arteries with low rates of stent thrombosis (ST) and target lesion revascularization (TLR).
Resorb
By degrading after fulfilling their scaffolding function, they offer all options of future therapies.
Product Highlights
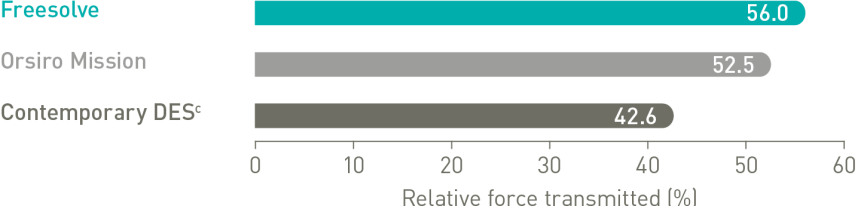
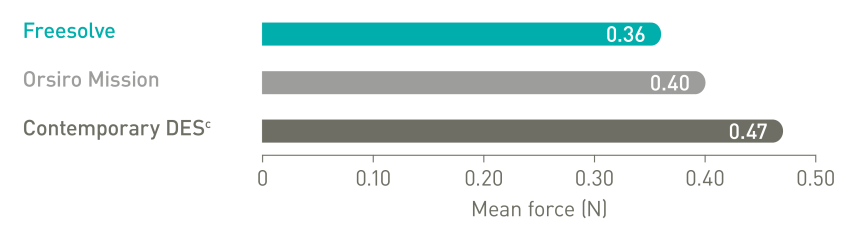
Delivers like a DES5
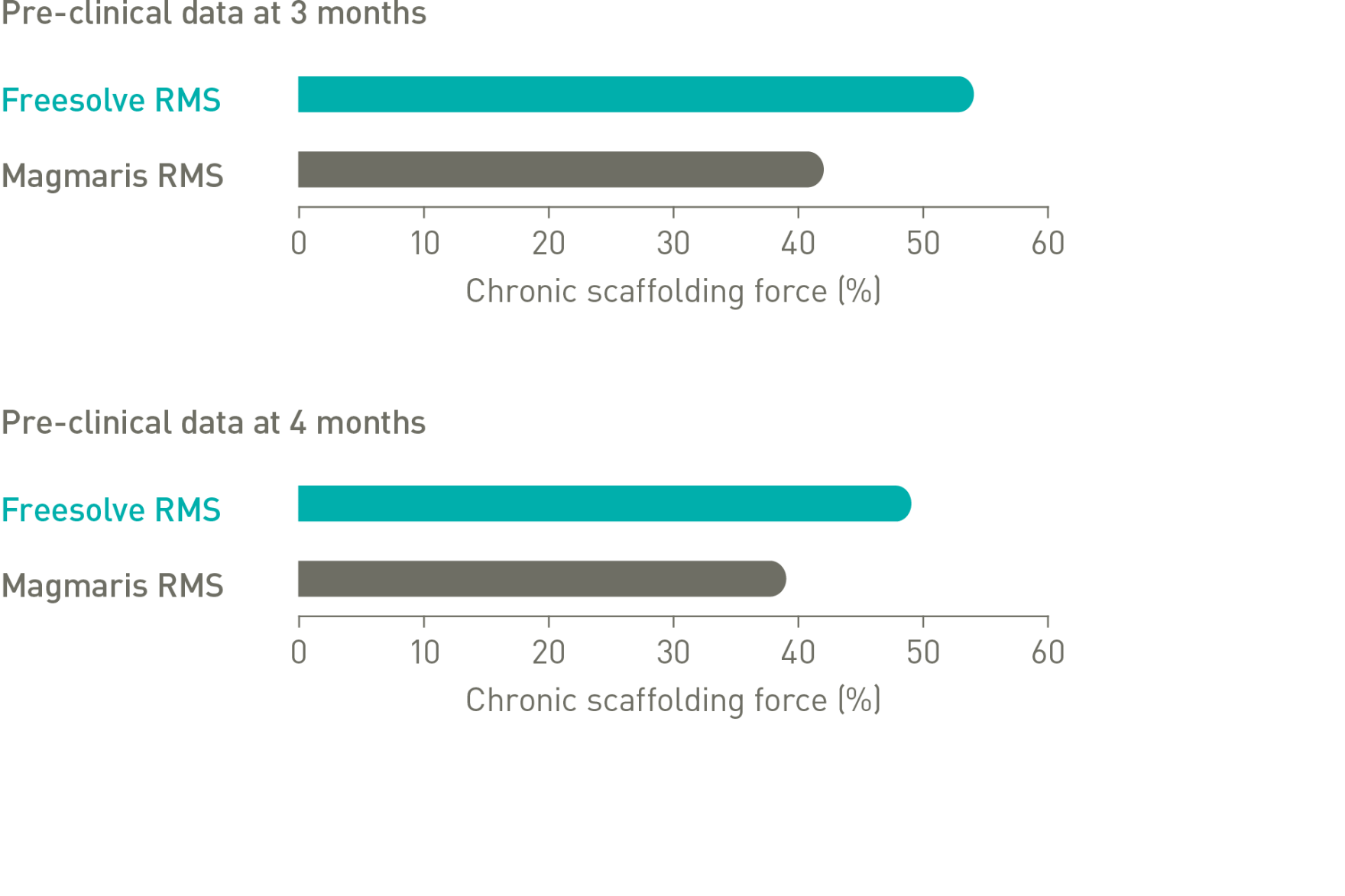
Optimal vessel support6,7
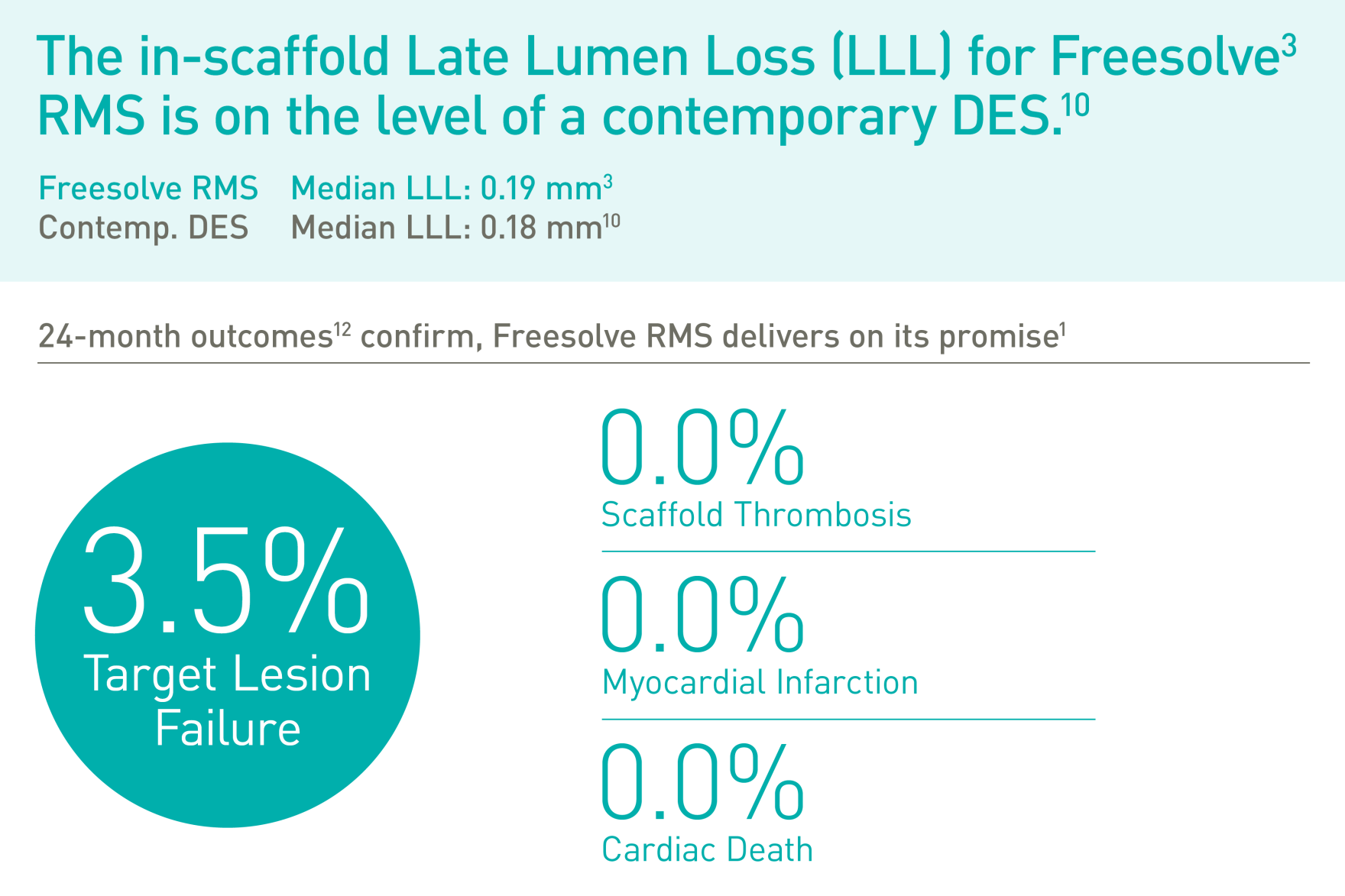
Magnesium fully resorbed after 12 months8
Excellent safety and efficacy2,3
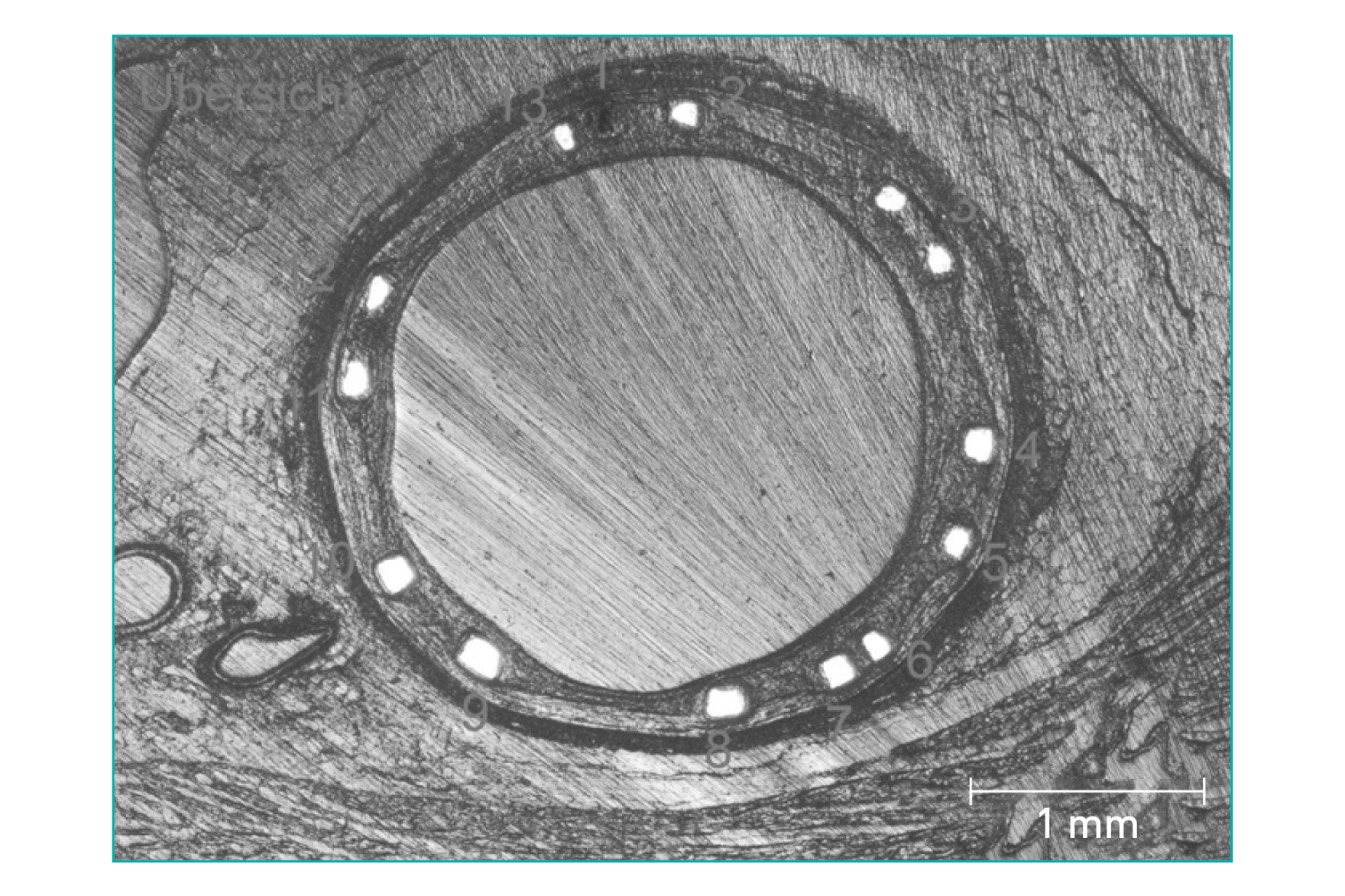
Predictable, homogenous resorption process⁶
Equal resorption between struts6
Uniform shape due to homogenous strut resorption6
More than 3 months vessel support6,7
>99% of struts no longer visible at 12 months8
BIOMAG-I First-In-Human (FIH) trial3
Product Overview
Freesolveᵀᴹ
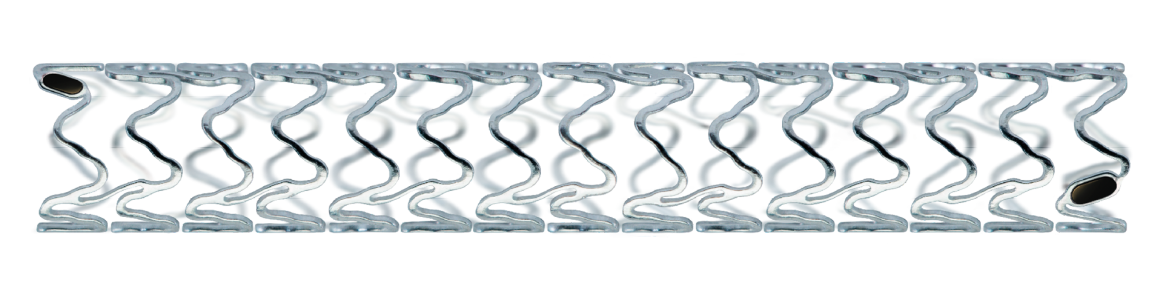
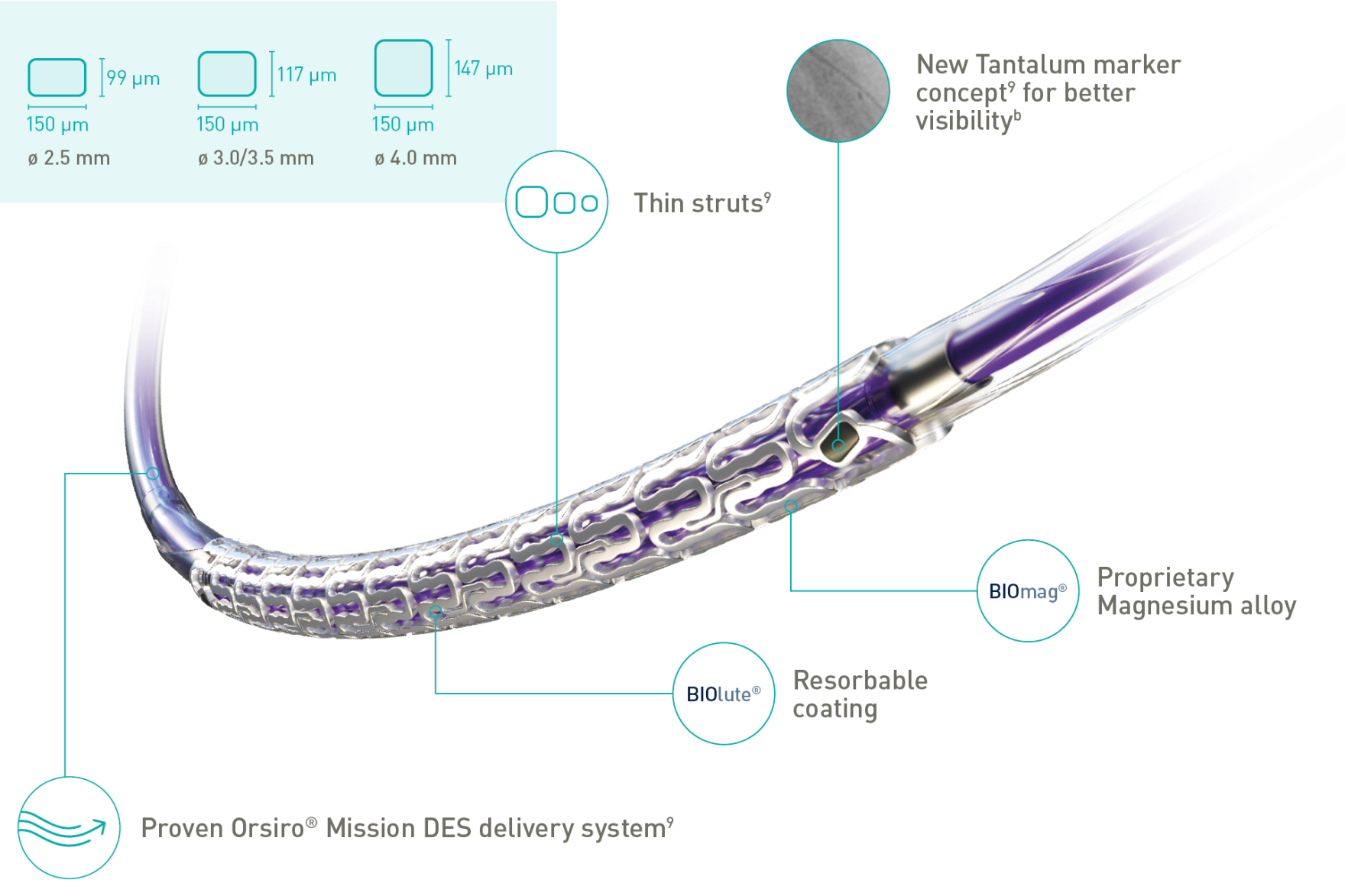
New Tantalum marker Concept for better visibility
Thin struts
BIOlute® Resorbable coating
BIOmag® Proprietary Magnesium alloy
Technical Data
| Scaffold | |||||
|---|---|---|---|---|---|
| Scaffold material | Proprietary BIOmag® Magnesium alloy | ||||
| Strut thickness | ø 2.5 mm: 99 μm; ø 3.0/3.5 mm: 117 μm; ø 4.0 mm: 147 μm |
||||
| Maximum expandable diameter | Nominal diameter + 0.6 mm | ||||
| Markers | One oval Tantalum marker at each end | ||||
| Drug coating | BIOlute® resorbable Poly-L-Lactide (PLLA) eluting a limus drug |
||||
| Delivery system | |||||
| Catheter type | Rapid exchange | ||||
| Catheter length | 140 cm | ||||
| Recommended guide catheter | 6F | ||||
| Crossing profile | ø 2.5 mm ≤ 1.3 mm; ø 3.0-4.0 mm ≤ 1.4 mm | ||||
| Guide wire diameter | 0.014” | ||||
| Nominal pressure (NP) | 10 atm | ||||
| Rate burst pressure (RBP) | 16 atm | ||||
Vessel Sizing
| Scaffold ø (mm) (SD) |
Recommended ø (mm) (RVD) |
|---|---|
| 2.50 | 2.50 - 2.70 |
| 3.00 | 2.70 - 3.20 |
| 3.50 | 3.20 - 3.70 |
| 4.00 | 3.70 - 4.20 |
Compliance Chart
| Balloon diameter (mm) | ||||
|---|---|---|---|---|
| ø 2.50 | ø 3.00 | ø 3.50 | ||
| Nominal Pressure (NP) |
atm** ø (mm) |
10 2.52 |
10 3.04 |
10 3.54 |
| Rated Burst Pressure (RBP) |
atm** ø (mm) |
16 2.72 |
16 3.29 |
16 3.79 |
| *1 atm = 1.013 bar | ||||
Ordering Information
| Scaffold ø (mm) |
Scaffold length (mm) |
||||
|---|---|---|---|---|---|
| 13 | 18 | 22 | 26 | 30 | |
| 2.50 | 443103 | 443104 | 443105 | - | - |
| 3.00 | 443108 | 443109 | 443110 | 482156 | 443111 |
| 3.50 | 443113 | 443114 | 443115 | 482157 | 443116 |
| 4.00 | 443118 | 443119 | 443120 | 482158 | 443121 |
Downloads and Related Links
Downloads
Related Links
Media
KOL Interviews
How can we help you?
References
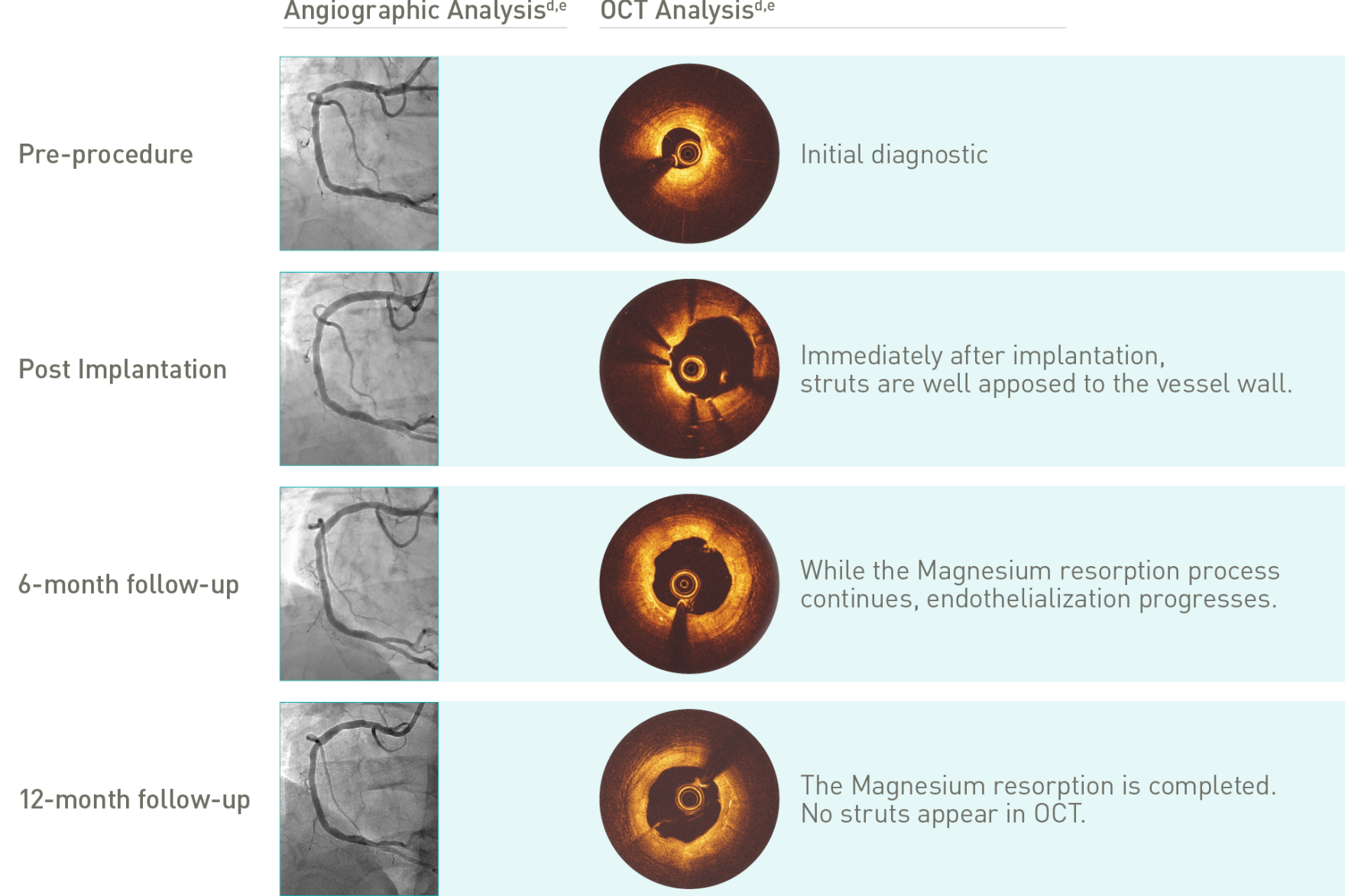
Target Lesion Failure (TLF) is a composite of Target-Vessel Myocardial Infarction (TV-MI), clinically-driven Target Lesion Revascularization (CD-TLR) and Cardiac Death. *99.3% resorbed at 12 months (markers are not resorbable), based on clinical data; **based on QCA paired data. a. Indications as per IFU; b. BIOMAG-I case in normal cine projection, courtesy of Prof. Michael Haude, Rheinland Klinikum Neuss GmbH, Lukaskrankenhaus, Neuss, Germany; c. Xience Sierra DES (Abbott); d. Angiographic and OCT Analyses derived from two different BIOMAG-I cases, courtesy of Prof. Michael Haude, Rheinland Klinikum Neuss GmbH, Lukaskrankenhaus, Neuss, Germany; e. The 4P protocol was respected.
1. IIB Benchtest data, BIOTRONIK data on file; 2. Haude M. et al., the Lancet eClinicalMedicine 2023;59: 101940; 3. Haude, M. et al., EuroIntervention 2023;19:1-1 published online May 2023; 4. Seguchi M et al. OCT-Analysis 12M, presented at ESC 2023; 5. BIOTRONIK data on file, IIB Benchtest data: Freesolve in comparison to BIOTRONIK Orsiro Mission and Abbott Xience Sierra; 6. Based on pre-clinical data, Seguchi, M. et al., EuroIntervention 2023;18-online publish-ahead-of-print January 2023; 7. BIOTRONIK data on file, in comparison to predecessor device; 8. Based on intravascular OCT analysis of the BIOMAG-I trial presented by Dr. M. Seguchi at ESC 2023; 9. BIOTRONIK data on file; 10. Byrne, RA. et al., Eur Heart J 2015;36:2608-2620; 11. Haude M., et al. Sustained safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de novo coronary lesions: 12-month clinical results and angiographic findings of the BIOSOLVE-II first-in-man trial. Eur Heart J. 2016;37:2701-9. 12. BIOMAG-I: two-year clinical outcomes of the resorbable magnesium Scaffold-DREAMS 3G, Moderated e-Poster presented by Prof. M. Haude at EuroPCR 2024.
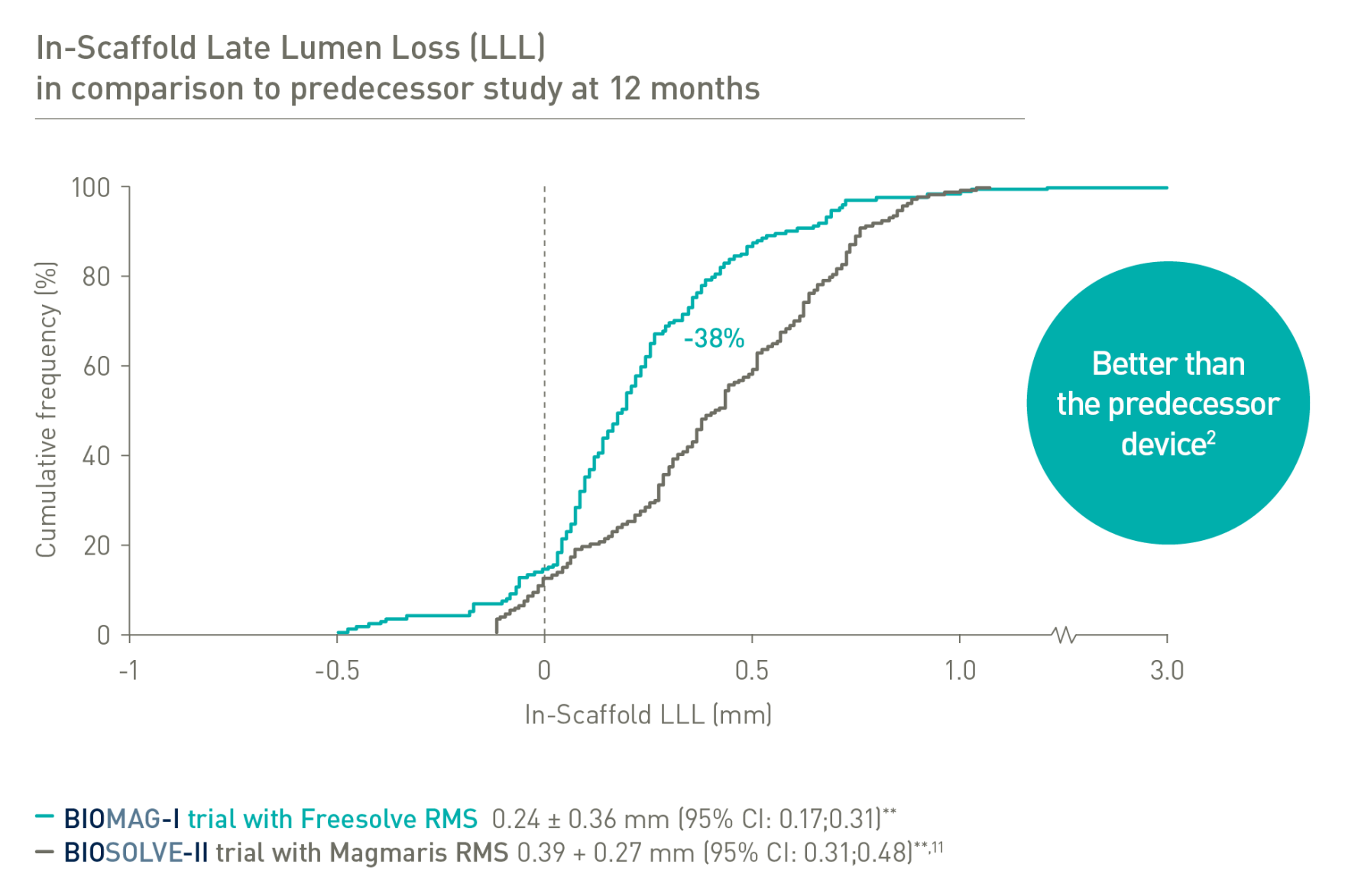
BIOSOLVE-II and BIOMAG-I based on Kaplan-Meier failure estimate analysis.
BIOlute, BIOmag, BIOMAG, BIOSOLVE, Orsiro, Orsiro Mission, Magmaris & Freesolve are trademarks or registered trademarks of the BIOTRONIK Group of Companies. All other trademarks are the property of their respective owner.