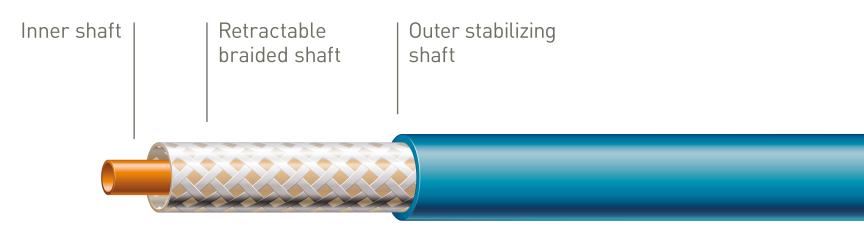
Unique tri-axial shaft design
The outer stabilizing shaft isolates the retractable shaft from friction caused by the introducer valve to ensure accurate stent deployment
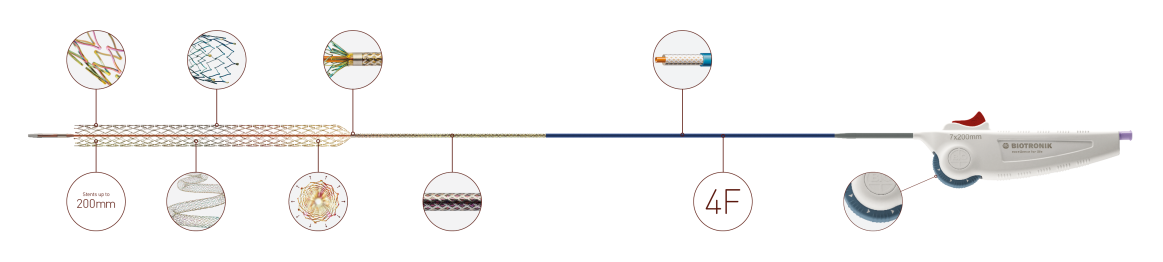
Pulsar-18 T3 is the first clinically-proven thin strut stent mounted on a tri-axial low profile (4F) delivery system. It is indicated for use in patients with atherosclerotic disease of the superficial femoral, proximal popliteal and infrapopliteal arteries and for the treatment of insufficient results after Percutaneous Transluminal Angioplasty (PTA), e.g. residual stenosis and dissection.*
The outer stabilizing shaft isolates the retractable shaft from friction caused by the introducer valve to ensure accurate stent deployment

| Stent | |
|---|---|
| Catheter type | OTW |
| Recommended guide wire | 0.018” |
| Stent material | Nitinol |
| Strut thickness | 140 μm |
| Strut width |
85 μm |
| Stent coating | proBIO® (Amorphous Silicon Carbide) |
| Stent markers | 6 gold markers each end |
| Sizes | ø 4.0 - 7.0 mm; L:20 - 200 mm |
| Shaft | 4F, hydrophobic coating, tri-axial |
| Usable length | 90 cm and 135 cm |
| Stent ø (mm) |
Catheter length 90 cm |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 20** | 30 | 40 | 60 | 80 | 100 | 120 | 150 | 170 | 200 | ||
| 4F | 4 | 430437 | 430438 | 430439 | 430440 | 430441 | 430442 | 430443 | 430444 | 430445 | 430446 |
| 5 | 430447 | 430448 | 430449 | 430450 | 430451 | 430452 | 430453 | 430454 | 430455 | 430456 | |
| 6 | 430457 | 430458 | 430459 | 430460 | 430461 | 430462 | 430463 | 430464 | 430465 | 430466 | |
| 7 | 430467 | 430468 | 430469 | 430470 | 430471 | 430472 | 430473 | 430474 | 430475 | 430476 | |
| **8 weeks pre-order only | |||||||||||
| Stent ø (mm) |
Catheter length 135 cm |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 20** | 30 | 40 | 60 | 80 | 100 | 120 | 150 | 170 | 200 | ||
| 4F | 4 | 430477 | 430478 | 430479 | 430480 | 430481 | 430482 | 430483 | 430484 | 430485 | 430486 |
| 5 | 430487 | 430488 | 430489 | 430490 | 430491 | 430492 | 430493 | 430494 | 430495 | 430496 | |
| 6 | 430497 | 430498 | 430499 | 430500 | 430501 | 430502 | 430503 | 430504 | 430505 | 430506 | |
| 7 | 430507 | 430508 | 430509 | 430510 | 430511 | 430512 | 430513 | 430514 | 430515 | 430516 | |
| **8 weeks pre-order only | |||||||||||
FTLR = Freedom from Target Lesion Revascularization.
1. BIOTRONIK data on file; 2. Zhao HQ. Late stent expansion and neointimal proliferation of oversized nitinol stents in peripheral arteries. Cardiovasc Intervent Radiol. 2009 Jul;32(4):720-6; 3. Bosiers M et al. 4-French – compatible endovascular material is safe & effective in the treatment of femoropopliteal occlusive disease: Results of the 4EVER Trial. ENDOVASC THER 2013; 20: 746-756; 4. Koskinas C. Role of endothelial shear stress in stent restenosis and thrombosis: pathophysiologic mechanisms and implications for clinical translation. JACC 2012 10;59(15):1337-49; 5. Koppara T. Thrombogenicity and early vascular healing response in metallic biodegradable polymer-based and fully bioabsorbable drug-eluting stents. Circ Cardiovasc Interv. 2015 8(6):e002427
*Indication as per IFU. Pulsar and proBIO are trademarks or registered trademarks of the BIOTRONIK Group of Companies.