1st in Pushability⁴
Transmitting up to 96% more force from hub to tip versus Resolute Onyx.
1st in Trackability⁴
Up to 33% less force needed to follow the path to the lesion versus Resolute Onyx.
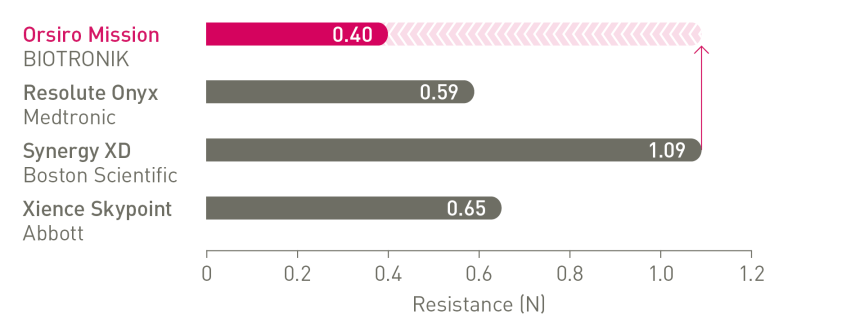
1st in Crossability⁴
Up to 64% less force needed to successfully cross demanding anatomies versus Synergy XD.