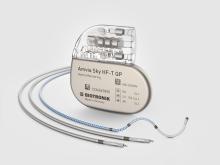
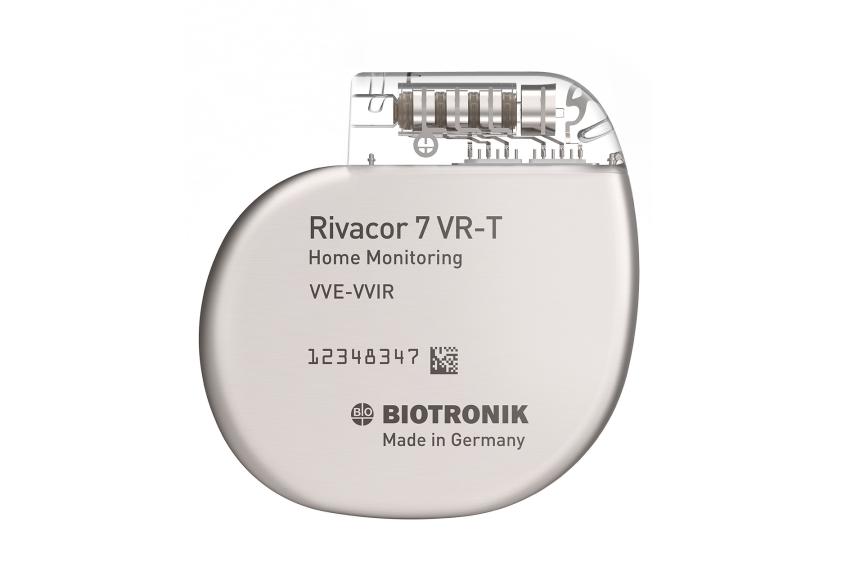
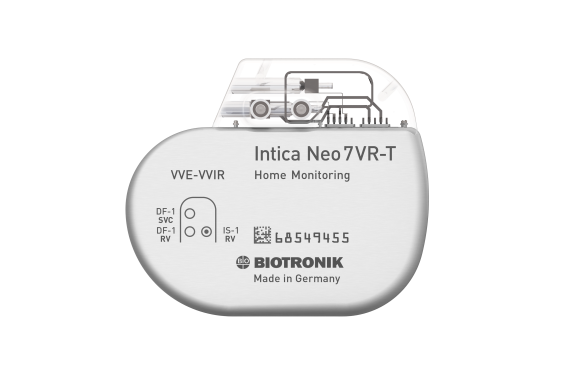
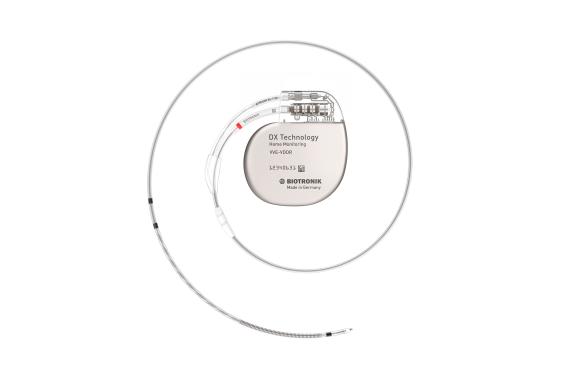
Rivacor 7 VR-T / DR-T
Improving treatment and reducing risk for up to 15 years1
For patients with arrhythmias, ICD therapy can be an essential safety net, where it’s vital to minimise risk and improve quality of life in the long term. This is what the all-new, smaller and simpler Rivacor 7 ICD systems are engineered to do for up to 15 years1 — optimising therapy when and where it matters. When used with Home Monitoring® technology, AF can be detected earlier2 and both inappropriate shocks and hospitalisation rates can be reduced3. And this has been clinically proven.
Product Highlights
BIOshape. Ultraslim 10mm ICD
Rivacor ICDs are small and slim – only 10mm from front to back – with a smooth, elliptical, and body-friendly BIOshape4
15 Years Longevity. 10 Years Warranty
Rivacor ICDs have an extended battery life of up to 15 years1, supported by a full 10-year warranty
DX Technology
All these benefits are also available with unique DX Technology that provides complete atrial diagnostics with a single lead
Downloads & Related Links
Contact Us
References
1. Single-Chamber ICD, Standard conditions, Rivacor 7 VR-T, RV: 2.5 V/0.4 ms, 40 bpm, 500 Ω; RV: 15 % pacing; 2 max. energy shocks/year; Home Monitoring: ON (daily transmission); diagnostics: ON. Data on file (Calculation of service times).; 2. Varma N et al. Efficacy and Safety of Automatic Remote Monitoring for Implantable Cardioverter-Defibrillator Follow-Up: The Lumos-T Safely Reduces Routine Office Device Follow-Up (TRUST) Trial. Circulation, 2010;122: 325 – 332.; 3. Guedon-Moreau L et al. Decreased delivery of inappropriate shocks achieved by remote monitoring of ICD: a substudy of the ECOST trial. J Cardiovasc Electrophysiol, 25 (2014), 763-770.; 4. Contoured housing; Acticor/Rivacor VR: 60x61.5x10 mm; 30 ccm.; 5. As part of an MR conditional system.; 6. Post-Market observation; Final report, March, 2019. Data on file.; 7. Device shape analysis, February 2019. Data on file.; 8. Post-Market observation; Interim-analysis, December 21, 2018. Data on file.; 9. Acticor/Rivacor Single-chamber ICD @ 60 ppm, 15% pacing, 2.5V, 500 Ohms: Medtronic 3T Full-body MRI; VISIA AF (EVERA; MIRRO) MRI S VR SureScan: 10.7 years (MDT IFU) vs Acticor 7 VR-T ProMRI: 14.9 years. Competitor device manuals as of Nov. 2018.; 10. Polyzos KA, Konstantelias AA, Falagas ME, Risk factors for cardiac implantable electronic device infection: A systematic review and meta-analysis, Europace (2015) 17, 767-777.; 11. In patients without a history of atrial arrhythmias at implant.; 12. Biffi M, Iori M, De Maria E, et al. The role of atrial sensing for new-onset atrial arrhythmias diagnosis and management in single-chamber implantable cardioverter-defibrillator recipients: Results from the THINGS registry. Journal of Cardiovascular Electrophysiology, Volume 31, Issue 4, April 2020, Pages 846–853.; 13. Pung X, Hong DZ, Ho TY, et al. The utilization of atrial sensing dipole in single lead implantable cardioverter defibrillator for detection of new-onset atrial high-rate episodes or subclinical atrial fibrillation: A systematic review and meta-analysis. Journal of Arrhythmia. Volume 38, Issue 2, 15 January 2022, Pages 177–186.; 14. Thomas G, Choi DY, Doppalapudi H, et al. Subclinical atrial fibrillation detection with a floating atrial sensing dipole in single lead implantable cardioverter-defibrillator systems: Results of the SENSE trial. Journal of Cardiovascular Electrophysiology, Volume 30, Issue 10, October 2019, Pages 1994–2001.; 15. Hindricks G, Theuns DA, Bar-Lev D, et al. Ability to remotely monitor atrial high-rate episodes using a single-chamber implantable cardioverter-defibrillator with a floating atrial sensing dipole, EP Europace, Volume 25, Issue 5, May 2023, Pages 1-10, euad061.; 16. Fact file: Cardiac Imaging with MRI, CT and Nuclear Techniques British Heart Foundation. January 2010.; 17. When patients are monitored by BIOTRONIK Home Monitoring. See ProMRI manual for all details.; 18. Schwab JO et al. Clinical Course of Dual-Chamber Implantable Cardioverter-Defibrillator Recipients followed by Cardiac Remote Monitoring: Insights from the LION Registry. BioMed Research International, 2018, https://doi.org/10.1155/2018/3120480.; 19. Ricci R P et al. Long-term patient acceptance of and satisfaction with implanted device remote monitoring. Europace (2010) 12, 674-679.; 20. Hindricks G et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. The Lancet. 2014; 384 (9943): 583–590.; 21. Crossley G H et al. The CONNEXT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) Trial. JACC. 2011; 57(10):1181-1189 [for bar chart comparison only].