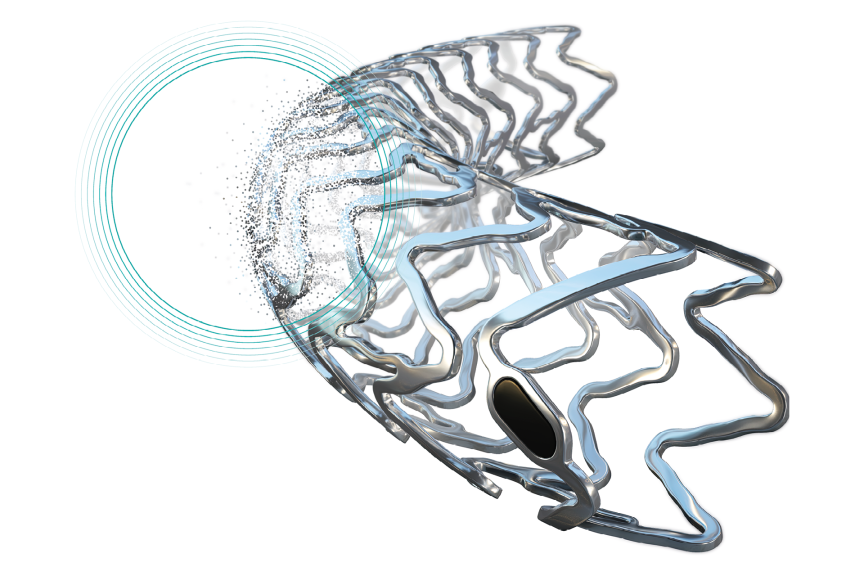
BIOTRONIK Introduces Expanded Maximum Diameter Dimensions for Orsiro Mission DES Globally Approved Maximum Diameter Expansion Aims to Provide New Options in Larger Diameter and Tapered Vessels
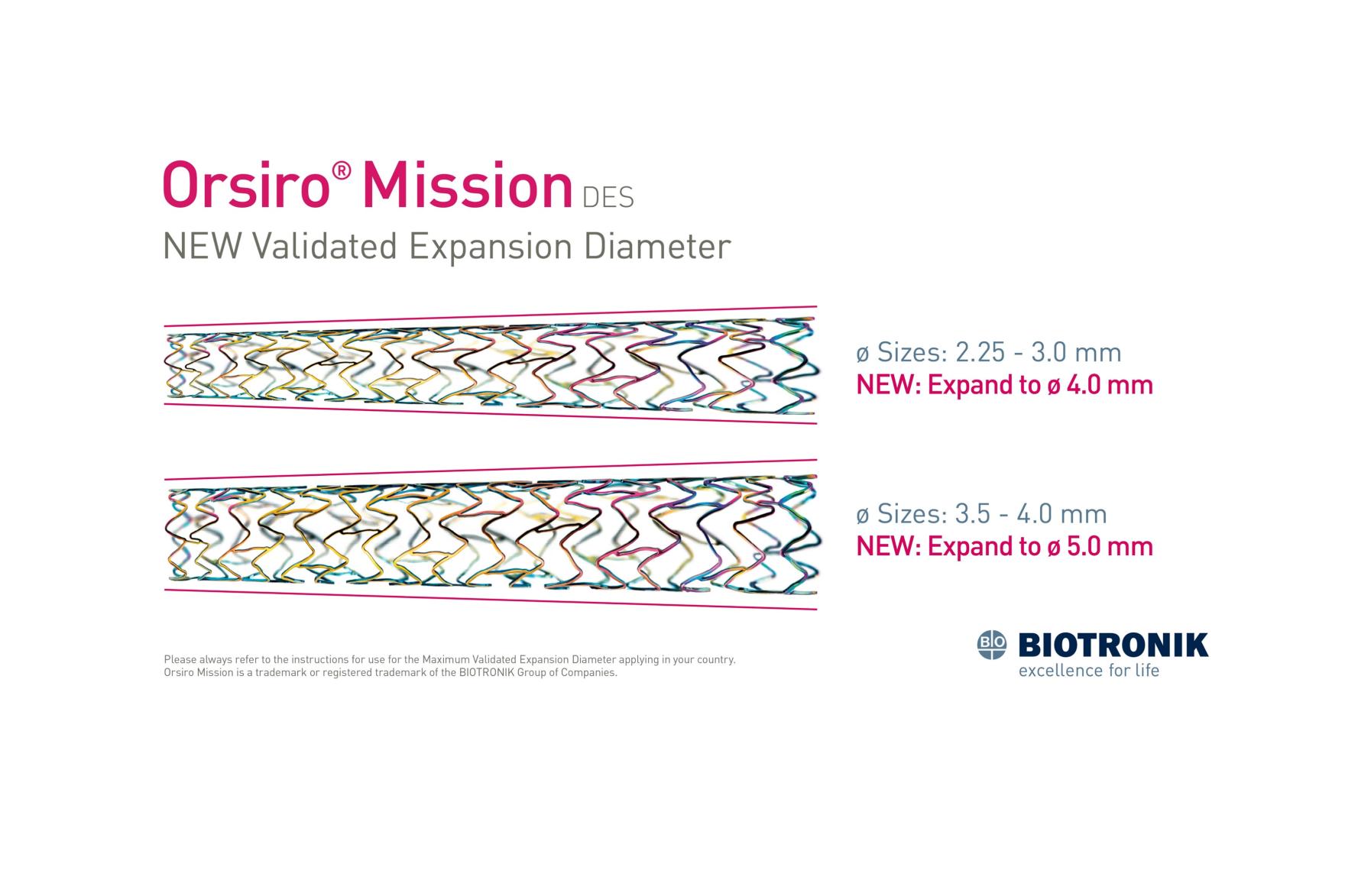
BIOTRONIK is pleased to announce the availability of an expanded Maximum Allowed Diameters (MAD) range for the Orsiro® Mission Drug Eluting Stent (DES). Diameters 2.25mm to 3.00mm of the Orsiro Mission DES can now be extended up to 4.0mm, while diameters 3.5mm and 4.0mm can reach up to 5.0mm.
This new MAD expansion follows the approvals from CE, FDA, and Health Canada obtained in the past months. This update reflects a global effort for the Orsiro Mission stent technology to match contemporary Percutaneous Coronary Intervention (PCI), enabling practitioners to optimize vessel apposition and conform to tapered anatomies.
Subsequently to these approvals, an updated labelling, additionally to the modified Instructions for Use (IFU), will provide further guidance to practitioners.
Always consult the Instructions for Use specific to your country when applying the Maximum Allowed Diameters (MAD).
More information on Orsiro® Mission DES here.
-END-
Disclaimer:
Orsiro and Orsiro Mission are trademarks or registered trademarks of the BIOTRONIK Group of Companies.
About BIOTRONIK:
At BIOTRONIK, patient well-being is our top priority and has been for 60 years. BIOTRONIK is a leading global medical technology company with products and services that save and improve the lives of millions suffering from heart and blood vessel diseases as well as chronic pain. Driven by a purpose to perfectly match technology with the human body, we are dedicated innovators who develop trusted cardiovascular, endovascular and neuromodulation solutions. BIOTRONIK is headquartered in Berlin, Germany, and is represented in over 100 countries.