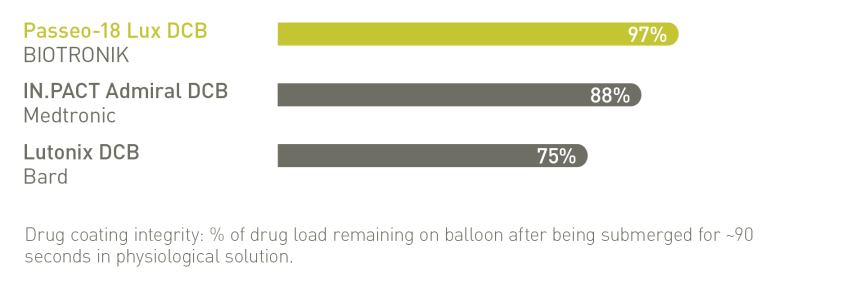
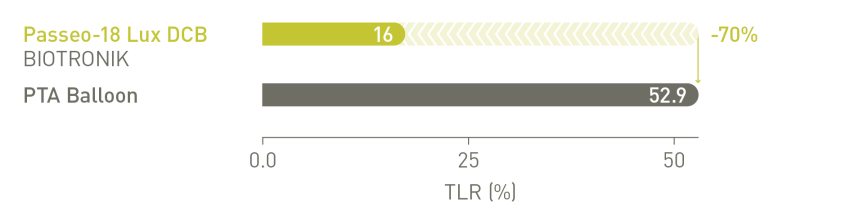
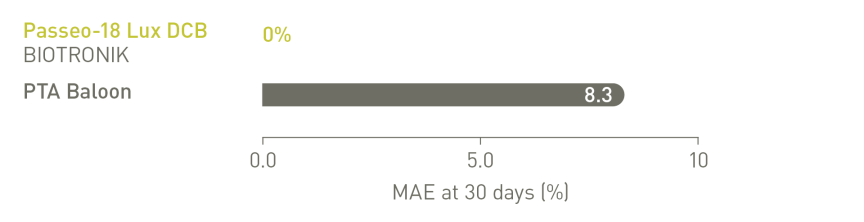BIOLUX P-I RCT² Femoropopliteal Indication¹⁰
Significantly reduced Target Lesion Revascularization (TLR) at 12 months compared
to the control PTA* balloon in the as-treated population²
BIOLUX P-II RCT³ Infrapopliteal Indication
Major Adverse Events (MAE) rate of the Passeo-18 Lux DCB was lower compared to
the control PTA balloon.
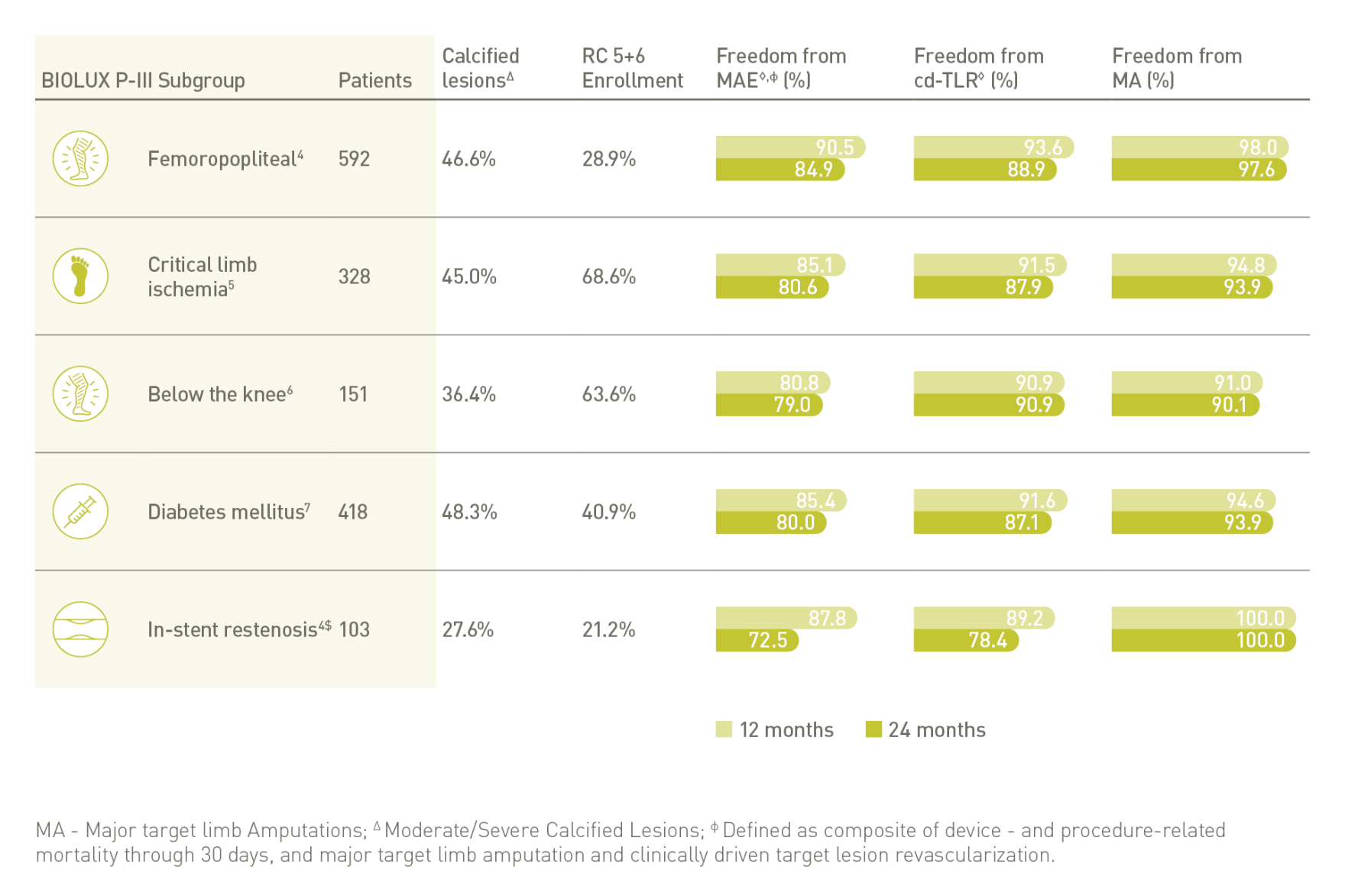
BIOLUX P-III⁴ All-Comers Registry
Passeo-18 Lux DCB demonstrates excellent outcomes in one of the largest real-world DCB registries with few exclusion criteria.