Dr. Koen Deloose, principal investigator of the BIO-OSCAR SOC, presenting the study outcomes at the Paris Vascular Insights Course 2024.
BIO-OSCAR SOC Trial to Examine Standard of Care in Peripheral Artery Disease Treatment Study to evaluate the baseline against which to measure the Oscar® Multifunctional Catheter in treating complex Peripheral Artery Disease (PAD)
BIOTRONIK has concluded the BIO-OSCAR SOC, a prospective, multicenter, observational trial. It is intended at evaluating current standard of care practices, procedural outcomes, complication handling and device use in endovascular treatments for atherosclerotic lesions both above the knee (ATK) and below the knee (BTK). The outcomes were presented today at the Paris Vascular Insights Course 2024 by Dr. Koen Deloose, principal investigator of the BIO-OSCAR SOC.
The study analyzed data from 247 patients across 16 European CE-mark territories, aiming to deliver insights into real-world practices for managing PAD.
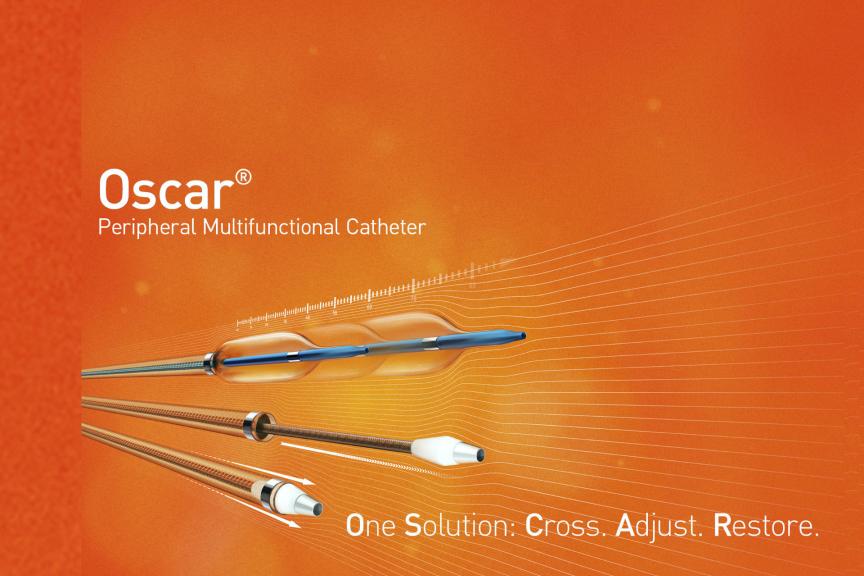
“BIO-OSCAR SOC is an important step toward understanding the treatment of complex infrainguinal lesions under current standard of care practices, and especially how important vessel preparation can be,” said Dr. Koen Deloose, Head of the Department of Vascular Surgery, AZ Sint Blasius Hospital in Dendermonde, Belgium. “With this baseline study, we aim to evaluate that the Oscar catheter can streamline procedures by minimizing the need for multiple device exchanges, reducing procedural steps, and lowering complications.”
The primary endpoint of BIO-OSCAR SOC was procedural success, defined as a combination of technical success — achieving ≤30% residual stenosis1 following vessel preparation and prior to the definitive treatment — and the absence of complications such as target vessel perforation, rupture, acute occlusion, or distal embolization.
The outcomes show:
- 50% procedural success1 in ATK and 59% in BTK1.
- 94% crossing success in Chronic Total Occlusions (CTO) for ATK1 and 90% for BTK1.
- 50% bail-out stenting rate in ATK cases1, highlighting that vessel preparation is often insufficient. Appropriate vessel preparation may significantly improve short and long-term outcomes1.
The procedural cost related to access, crossing and vessel preparation averages €750, while for complex cases the cost can go up to €11001.
The clinical evidence will be further validated by the BIO-OSCAR FIRST study, which is designed to confirm the safety and clinical performance of the Oscar® Peripheral Multifunctional Catheter for dilation of lesions in the femoral, popliteal, and infrapopliteal arteries2, including both ATK and BTK lesions.
“This study provides the vascular community with valuable insights into existing procedural success rates and cost efficiency data,” says Stuart Perks, Vice President Marketing at BIOTRONIK Vascular Intervention. “BIO-OSCAR SOC is pivotal in validating Oscar’s role in improving the standard of care for PAD and optimizing treatment strategies for patients with complex lesions."
The BIO-OSCAR SOC study establishes the baseline of standard of care, which will be used to evaluate the outcomes of the BIO-OSCAR FIRST study, emphasizing the effectiveness of the Oscar multifunctional catheter.
-END-
References:
1 Deloose K., Evaluation of Standard of Care Practices for Treatment of Infrainguinal Arteries Lesions presented at PVI Course 2024, December 2024, Paris, France
2 See Instruction for Use
Disclaimer: Oscar is a trademark or registered trademark of the BIOTRONIK Group of Companies.
For more information, please visit: Oscar
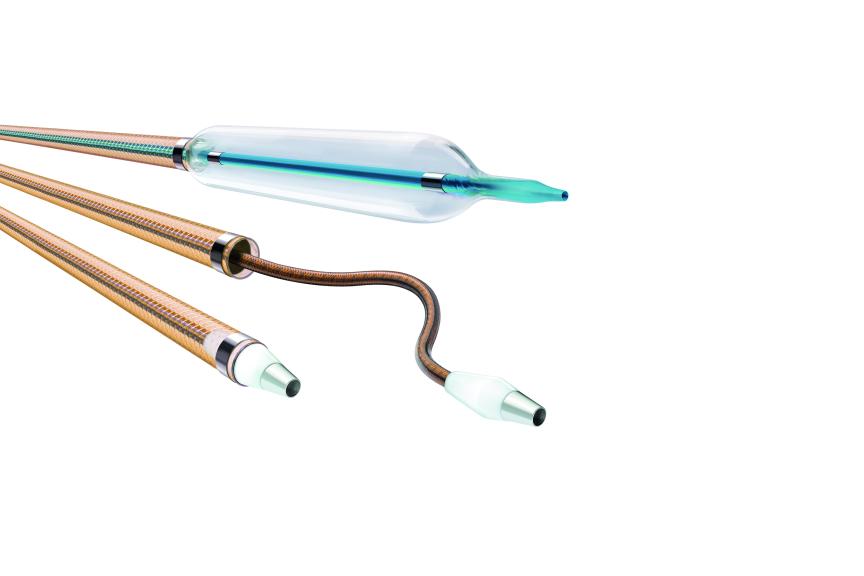
Oscar® Multifunctional Catheter is indicated for percutaneous transluminal interventions in the peripheral vasculature to provide support during access into and to dilate stenoses in femoral, popliteal and infrapopliteal arteries. The product is also intended for injection of radiopaque contrast media for the purpose of angiography.
About BIOTRONIK
At BIOTRONIK, patient well-being is our top priority and has been for over 60 years. BIOTRONIK is a leading global medical technology company with products and offerings that save and improve the lives of millions suffering from heart and blood vessel diseases as well as chronic pain. Driven by a purpose to perfectly match technology with the human body, we are dedicated innovators who develop trusted cardiovascular, endovascular and neuromodulation therapies. BIOTRONIK is headquartered in Berlin, Germany, and is represented in over 100 countries across the Americas, EMEA (Europe, the Middle East, and Africa), and Asia-Pacific.