Late-Breaking Study Data: BIOTRONIK’s Orsiro DES Outperforms Other Ultrathin Strut Drug-Eluting Stent A Subgroup Analysis of the HOST-IDEA Randomized Controlled Trial Showed Efficacy Differences of Ultrathin Strut DES
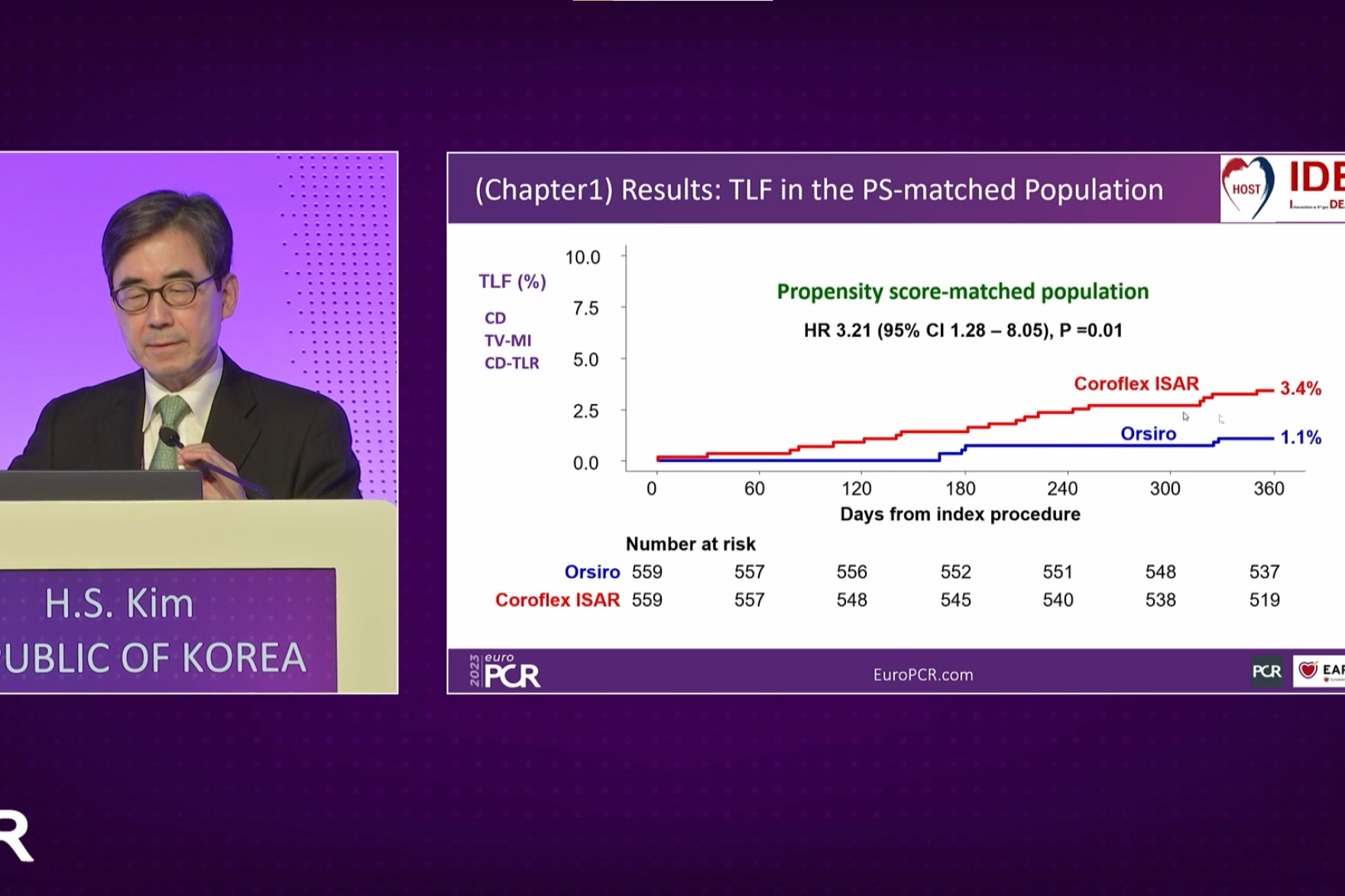
In a late breaking trial session during EuroPCR 2023 in Paris, on behalf of the HOST-IDEA study investigators, Dr. Hyo-Soo Kim presented the results of a stent level analysis comparing two ultrathin strut drug-eluting stents (DES): Orsiro® and Coroflex ISAR. The post-hoc comparison revealed significant differences in efficacy.
HOST-IDEA is a large scale, multicenter, all-comers randomized controlled trial that demonstrated the non-inferiority of 3- to 6-month versus 12-month dual antiplatelet therapy (DAPT) after implantation of ultrathin strut DES. 2,173 patients in 37 South Korean centers were enrolled and treated either with Coroflex ISAR DES (n=559) or Orsiro DES (n=1,449). The study outcomes were published in Circulation1.
In the presented post-hoc analysis out of a propensity score-matched population, results have shown that2:
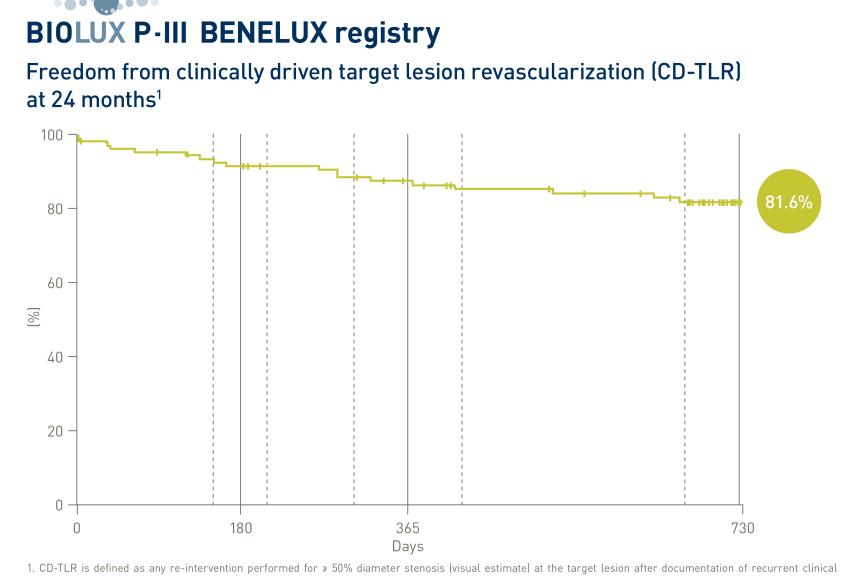
Dr. Kim also shared additional findings from the post-hoc analysis of the clinical performance of the two devices following either 3- or 12-month DAPT duration post percutaneous coronary intervention. Results have shown that the Orsiro DES performed better for both DAPT duration scenarios providing especially at three months 1.5% TLF versus 3.0% in the case of the Coroflex ISAR DES.
“Our post-hoc analysis showed that Orsiro biodegradable polymer sirolimus-eluting stent was associated with significantly better clinical outcomes than the Coroflex ISAR polymer-free sirolimus-eluting stent, mainly due to a lower rate of CD-TLR,” concluded Dr. Hyo-Soo. Kim, Interventional Cardiologist at Seoul National University Hospital, South Korea. “The current results indicate that third-generation DES with ultrathin struts are not all the same – probably due to the differences in release kinetics and sirolimus dosage. The results are important to guide future directions of the development of DES.”
“When optimizing the care path for patients, short-DAPT duration is a critical concern for clinicians. BIOTRONIK continues to build solid evidence in the Orsiro DES family range clinical program, which has now more than 71,000 patients enrolled4,” commented Stuart Perks, Vice President Marketing Vascular Intervention at BIOTRONIK. “The HOST-IDEA study outcomes continue to show that not all DES perform equally, especially in the ultrathin strut DES arena, where no class effect can be assumed for clinical performance.”
- END -
References:
1. Han J.K.et al. Comparison of 3- to 6-Month Versus 12-Month Dual Antiplatelet Therapy After Coronary Intervention Using the Contemporary Drug-Eluting Stents With Ultrathin Struts: The HOST-IDEA Randomized Clinical Trial, Circulation, 2023.
2. Kim H.-S., Biodegradable polymer vs. polymer-free ultrathin sirolimus-eluting stents, Presented at EuroPCR, Paris, 2023 (https://www.crtonline.org/presentation-detail/new-powerpoint-108)
3. Target lesion failure is a combined endpoint of cardiac death, target vessel related myocardial infarction, and clinically driven target lesion revascularization.
4. BIOTRONIK data on file
Disclaimer:
Orsiro is a trademark or registered trademark for the BIOTRONIK Group of Companies. All other trademarks are the property of their respective owners.
For more information, visit: www.orsiro.com
About BIOTRONIK:
At BIOTRONIK, patient well-being is our top priority and has been for 60 years. BIOTRONIK is a leading global medical technology company with products and services that save and improve the lives of millions suffering from heart and blood vessel diseases as well as chronic pain. Driven by a purpose to perfectly match technology with the human body, we are dedicated innovators who develop trusted cardiovascular, endovascular and neuromodulation solutions. BIOTRONIK is headquartered in Berlin, Germany, and is represented in over 100 countries.