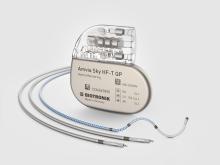
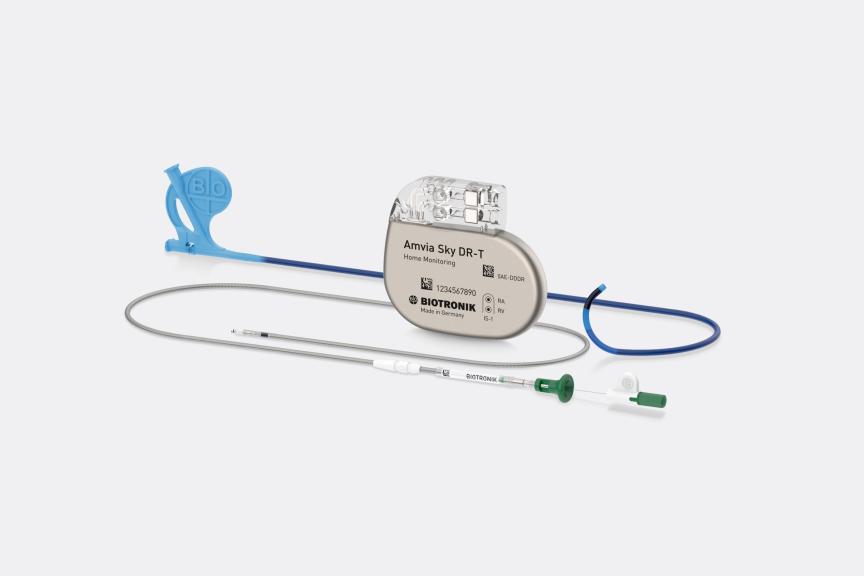
Amvia Sky Launched in Canada, the World’s First Pacemaker Approved for LBBAP* First Implant of Amvia Sky in Canada Performed at the Centre Hospitalier de l'Université de Montréal
Dr. Fadi Mansour performed the first Canadian implant of BIOTRONIK’s newest pacemaker and CRT-P generation earlier this year at the Centre Hospitalier de l'Université de Montréal. The patient received an Amvia Sky HF-T QP triple chamber pacemaker device. The first implant follows the full market release of BIOTRONIK’s newest family of pacemaker and CRT-P devices, featuring patient-centric technologies for better patient care and simplified workflows.
“The Amvia Sky HF-T QP offers a significant number of advanced features, making it the most complete CRT-P on the market including streamlined care pathways,” commented Dr. Mansour after the procedure.
The Amvia Sky system offers the following new features:
- Industry-leading choice with 20 left ventricular (LV) pacing polarities, automatically tested in less than 2.5 minutes.
- CRT AutoAdapt which automatically adjusts to changes in the patient’s condition for a more personalized response.
- Enhanced Closed Loop Stimulation (CLS) rate adaptation technology.
- Atrial arrhythmia management tools, including atrial antitachycardia pacing (ATP).
- Streamlined Care Pathways.
- Next-generation MRI access with MRI Guard 24/7.
“BIOTRONIK is dedicated to continually improving our technologies, and the Amvia Sky family is a great example of this commitment,” said Angela McGonigle, Country Director BIOTRONIK Canada. “Our latest family of pacemaker and CRT-P devices offers advanced features for optimized therapy and improved workflow simplification for Allied Health Professionals.”
-END-
References:
1.* BIOTRONIK Amvia Sky technical manual, Medtronic Azure XT DR MRI SureScan™ manual; Boston Scientific Accolade MRI™ technical manual; Abbott Assurity MRI™ user’s manual; MicroPort Alizea™ implant manual.
2. Garcia-Fernández FJ, Asensi JO, Romero R, et al. Safety and efficiency of a common and simplified protocol for pacemaker and defibrillator surveillance based on remote monitoring only: a long-term randomized trial (RM-ALONE). Eur Heart J. 2019; 40(23): 1837–1846.
3. Data on file.
4. Mullane S, Michaelis K, Henrickson C, et al. Utilization and programming of an automatic MRI recognition feature for cardiac rhythm management devices. Heart Rhythm. O2. 2021; 2: 132–137.
About BIOTRONIK:
At BIOTRONIK, patient well-being is our top priority and has been for 60 years. BIOTRONIK is a leading global medical technology company with products and services that save and improve the lives of millions suffering from heart and blood vessel diseases as well as chronic pain. Driven by a purpose to perfectly match technology with the human body, we are dedicated innovators who develop trusted cardiovascular, endovascular and neuromodulation solutions. BIOTRONIK is headquartered in Berlin, Germany, and is represented in over 100 countries.